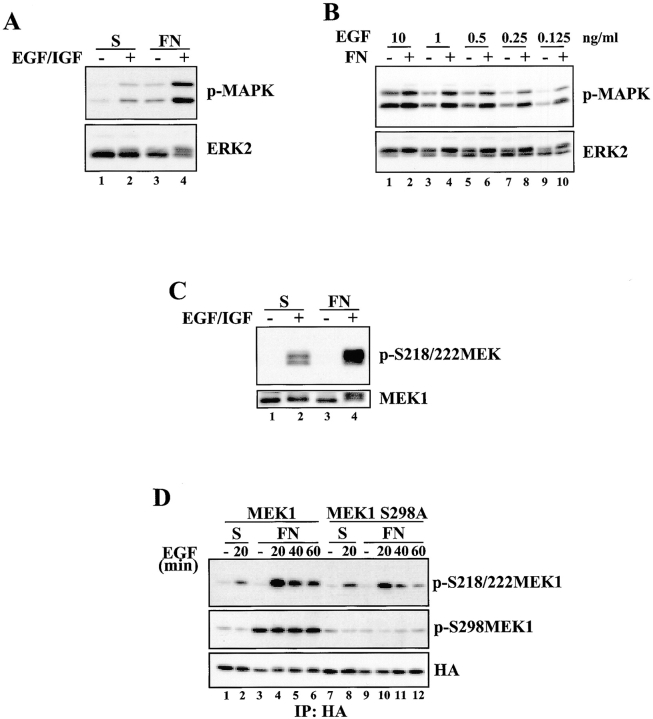
Figure 10.
Adhesion-dependent MEK1 S298 phosphorylation promotes maximal MEK1 activation in response to growth factor stimulation. (A) REF52 cells were suspended for 90 min and either stimulated for 30 min with EGF and IGF-1 in suspension, replated on FN for 30 min, or stimulated with EGF and IGF-1 while they attached to FN for 30 min. Whole cell lysates were blotted with p-MAPK or ERK2 antisera. (B) REF52 cells were placed in suspension for 90 min and either stimulated in suspension for 30 min with the indicated concentrations of EGF or replated on FN in the presence of the indicated concentrations of EGF for 30 min. Whole cell lysates were blotted with p-MAPK or ERK2 antisera. (C) Cells were treated as in A, and whole cell lysates were blotted with p-S218/222 MEK or MEK1 antisera. (D) REF52 cells were transiently transfected with HA-MEK1 (lanes 1–6) or HA-MEK1 S298A (lanes 7–12). Cells were suspended in serum-free media for 90 min and either kept unstimulated in suspension (lanes 1 and 7), stimulated in suspension with EGF for 20 min (lanes 2 and 8), allowed to adhere to FN (lanes 3 and 9) or allowed to attach to FN while stimulated with EGF for the indicated times (lanes 4–6 and 10–12). Anti-HA immunoprecipitates were formed and blotted with p-S218/222 MEK1, p-S298MEK1 or HA antisera. Densitometry and normalization to the loading controls revealed that S218/222 phosphorylation of MEK1 S298A was ∼50% that seen in the wild-type protein.