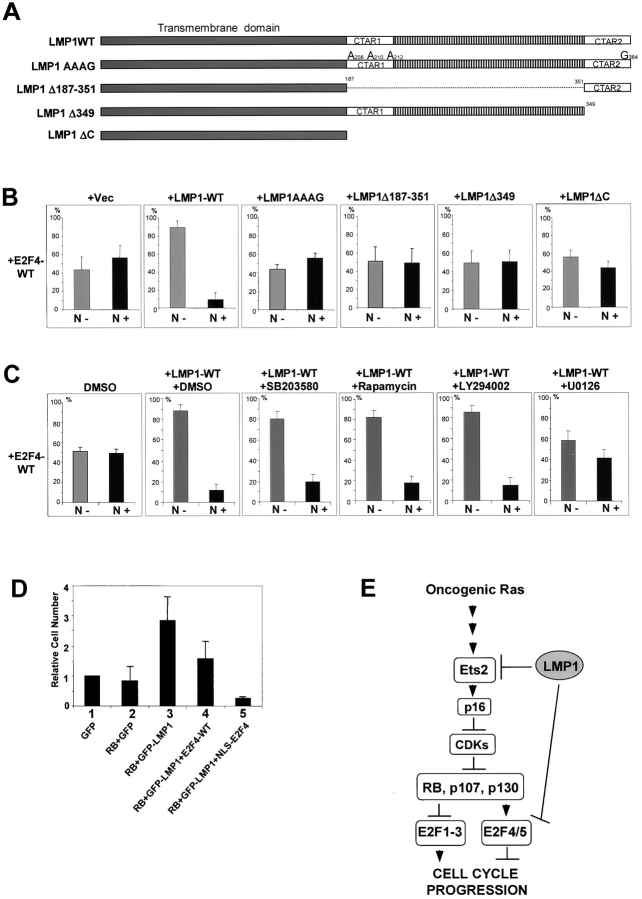
Figure 7.
Both CTAR1/2 domains are required for the nuclear export of E2F4. (A) Schematic representation of LMP1 mutants used for assay described in B. (B) Wild-type E2F4 was coexpressed with wild-type and a series of LMP1 mutants in SVts8 cells. E2F4 was detected by immunostaining with anti-E2F4 antibody and the histograms indicating the percentage of nuclei that were positive (N+) or negative (N−) for E2F4 expression were shown. (C) Wild-type E2F4 was coexpressed with LMP1 in the presence or absence of pharmacological inhibitors (SB203580, Rapamycin, LY294002, and U0126) at 10 μM, 0.1 nM, 20 μM, and 10 μM, respectively. 2 d after the transfection, E2F4 was detected by immunostaining with anti-E2F4 antibody, and the histograms indicating the percentage of nuclei that were positive (N+) or negative (N−) for E2F4 expression were shown. (D) Early passage (40 PDLs) TIG-3 cells were transfected with plasmids shown at the bottom by Nucleofector primary cell transfection system (Amaxa Biosystems). Transfection efficiencies were nearly 100% in all the transfected cells. Relative cell numbers were calculated using the GFP-transfected cells (lane 1) as the reference after 3 d. The experiments were repeated eight times with similar results. (B–D) Error bars indicate SD. (E) Model of LMP1 effects on p16INK4a–pRB pathway. Oncogenic Ras/MAPK signaling activates Ets2 and p16INK4a induction, thereby causing growth arrest/cellular senescence. Expression of the LMP1 oncoprotein blocks this pathway by targeting Ets2 and E2F4/5 for CRM1-dependent intracellular relocation.