Figure 5.
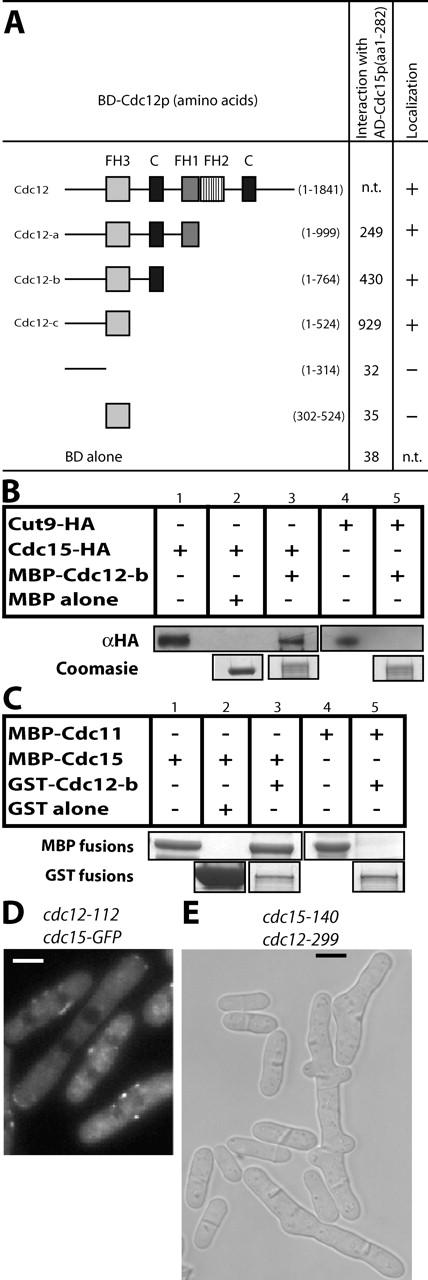
Cdc15p interacts directly with Cdc12p. (A) The indicated regions of Cdc12p were tested for interaction with Cdc15p (amino acids 1–282) by two-hybrid analysis. LEU+ TRP+ transformants were tested for growth on selective media (not depicted) and assayed for β-galactosidase activity measured in relative light units. GFP fusions of the cdc12 constructs were also expressed under control of the nmt81 promoter and visually examined for their ability (+) or lack of ability (−) to localize to an interphase spot structure and also to the CAR (column 3). (B) MBP (lane 2) or MBP–Cdc12p-b (amino acids 1–764) (lanes 3 and 5) both bound to amylose beads were incubated with protein lysates from either a cdc15-HA strain (KGY3020) or a cut9-HA strain (KGY1463) and subsequently extensively washed in binding buffer. Bound proteins were then divided and analyzed by immunoblotting (top) and Coomassie staining (bottom). Only relevant portions of the gels are shown; however, all proteins ran at the predicted sizes. (C) GST (lane 2, bottom) or GST–Cdc12-b (lanes 3 and 5, bottom) both bound to glutathione beads were mixed with either soluble MBP–Cdc15p(1–282) (lanes 1–3, top) or soluble MBP–Cdc11p(1–660) (lanes 4 and 5, top). Proteins were resolved by SDS-PAGE and detected by Coomassie staining. Only relevant portions of the Coomassie gel are shown to indicate loading; however, all proteins ran at the predicted sizes. Lanes 1 and 4 contain samples of the MBP fusion proteins before the binding reactions. (D) Localization of Cdc15p–GFP in cdc15-GFP cdc12-112 cells after 4 h at 36°C. (E) Phase contrast image of cdc15-140 cdc12-299 cells grown at 25°C. Bars, 5 μm.
