Abstract
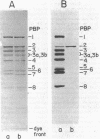
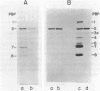
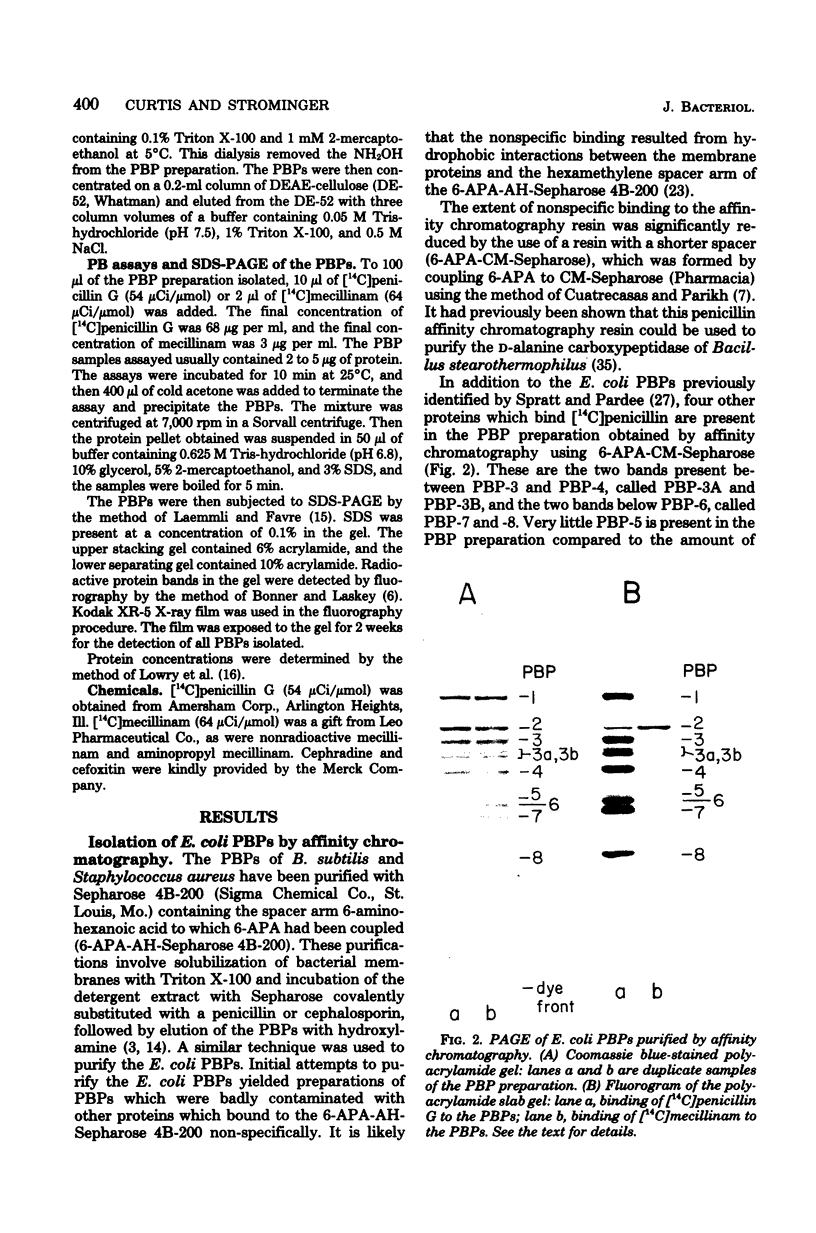
Penicillin-binding protein 2 (PBP-2) of Escherichia coli K-12 was purified by covalent affinity chromatography using 6-aminopenicillanic acid covalently coupled to carboxymethyl-Sepharose (6-APA-CM-Sepharose). Purification of PBP-2 was accomplished by prebinding the methoxy cephalosporin, cefoxitin, to the Triton X-100-solubilized PBPs of E. coli and then incubating the PBPs with 6-APA-CM-Sepharose. Cefoxitin readily binds to all the E. coli PBPs except PBP-2 and, thus, in the presence of cefoxitin, only PBP-2 could bind to the 6-APA-CM-Sepharose. The purification of a mixture of all of the PBPs of E. coli by affinity chromatography is also described.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blumberg P. M. Penicillin binding components of bacterial cells and their relationship to the mechanism of penicillin action. Ann N Y Acad Sci. 1974 May 10;235(0):310–325. doi: 10.1111/j.1749-6632.1974.tb43274.x. [DOI] [PubMed] [Google Scholar]
- Blumberg P. M., Strominger J. L. Inactivation of D-alanine carboxypeptidase by penicillins and cephalosporins is not lethal in Bacillus subtilis. Proc Natl Acad Sci U S A. 1971 Nov;68(11):2814–2817. doi: 10.1073/pnas.68.11.2814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumberg P. M., Strominger J. L. Interaction of penicillin with the bacterial cell: penicillin-binding proteins and penicillin-sensitive enzymes. Bacteriol Rev. 1974 Sep;38(3):291–335. doi: 10.1128/br.38.3.291-335.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumberg P. M., Strominger J. L. Isolation by covalent affinity chromatography of the penicillin-binding components from membranes of Bacillus subtilis. Proc Natl Acad Sci U S A. 1972 Dec;69(12):3751–3755. doi: 10.1073/pnas.69.12.3751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumberg P. M., Yocum R. R., Willoughby E., Strominger J. L. Binding of (14C)penicillin G to the membrane-bound and the purified D-alanine carboxypeptidases from Bacillus stearothermophilus and Bacillus subtilis and its release. J Biol Chem. 1974 Nov 10;249(21):6828–6835. [PubMed] [Google Scholar]
- Cuatrecasas P., Parikh I. Adsorbents for affinity chromatography. Use of N-hydroxysuccinimide esters of agarose. Biochemistry. 1972 Jun 6;11(12):2291–2299. doi: 10.1021/bi00762a013. [DOI] [PubMed] [Google Scholar]
- Gennis R. B., Strominger J. L. Activation of C55-isoprenoid alcohol phosphokinase from Staphylococcus aureus. III. Activation by detergents. J Biol Chem. 1976 Mar 10;251(5):1277–1282. [PubMed] [Google Scholar]
- Iwaya M., Goldman R., Tipper D. J., Feingold B., Strominger J. L. Morphology of an Escherichia coli mutant with a temperature-dependent round cell shape. J Bacteriol. 1978 Dec;136(3):1143–1158. doi: 10.1128/jb.136.3.1143-1158.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwaya M., Jones C. W., Khorana J., Strominger J. L. Mapping of the mecillinam-resistant, round morphological mutants of Escherichia coli. J Bacteriol. 1978 Jan;133(1):196–202. doi: 10.1128/jb.133.1.196-202.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwaya M., Strominger J. L. Simultaneous deletion of D-alanine carboxypeptidase IB-C and penicillin-binding component IV in a mutant of Escherichia coli K12. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2980–2984. doi: 10.1073/pnas.74.7.2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izaki K., Matsuhashi M., Strominger J. L. Glycopeptide transpeptidase and D-alanine carboxypeptidase: penicillin-sensitive enzymatic reactions. Proc Natl Acad Sci U S A. 1966 Mar;55(3):656–663. doi: 10.1073/pnas.55.3.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izaki K., Strominger J. L. Biosynthesis of the peptidoglycan of bacterial cell walls. XIV. Purification and properties of two D-alanine carboxypeptidases from Escherichia coli. J Biol Chem. 1968 Jun 10;243(11):3193–3201. [PubMed] [Google Scholar]
- Kozarich J. W., Strominger J. L. A membrane enzyme from Staphylococcus aureus which catalyzes transpeptidase, carboxypeptidase, and penicillinase activities. J Biol Chem. 1978 Feb 25;253(4):1272–1278. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K., Favre M. Maturation of the head of bacteriophage T4. I. DNA packaging events. J Mol Biol. 1973 Nov 15;80(4):575–599. doi: 10.1016/0022-2836(73)90198-8. [DOI] [PubMed] [Google Scholar]
- Lund F., Tybring L. 6 -amidinopenicillanic acids--a new group of antibiotics. Nat New Biol. 1972 Apr 5;236(66):135–137. doi: 10.1038/newbio236135a0. [DOI] [PubMed] [Google Scholar]
- Matsuhashi M., Maruyama I. N., Takagaki Y., Tamaki S., Nishimura Y., Hirota Y. Isolation of a mutant of Escherichia coli lacking penicillin-sensitive D-alanine carboxypeptidase IA. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2631–2635. doi: 10.1073/pnas.75.6.2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuhashi M., Takagaki Y., Maruyama I. N., Tamaki S., Nishimura Y., Suzuki H., Ogino U., Hirota Y. Mutants of Escherichia coli lacking in highly penicillin-sensitive D-alanine carboxypeptidase activity. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2976–2979. doi: 10.1073/pnas.74.7.2976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuhashi M., Tamaki S., Curtis S. J., Strominger J. L. Mutational evidence for identity of penicillin-binding protein 5 in Escherichia coli with the major D-alanine carboxypeptidase IA activity. J Bacteriol. 1979 Jan;137(1):644–647. doi: 10.1128/jb.137.1.644-647.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuhashi S., Kamiryo T., Blumberg P. M., Linnett P., Willoughby E., Strominger J. L. Mechanism of action and development of resistance to a new amidino penicillin. J Bacteriol. 1974 Feb;117(2):578–587. doi: 10.1128/jb.117.2.578-587.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melchior N. H., Blom J., Tybring L., Birch-Andersen A. Light and electron microscopy of the early response of Escherichia coli to a 6beta-amidinopenicillanic acid (FL 1060). Acta Pathol Microbiol Scand B Microbiol Immunol. 1973 Aug;81(4):393–407. doi: 10.1111/j.1699-0463.1973.tb02222.x. [DOI] [PubMed] [Google Scholar]
- O'Carra P., Barry S., Griffin T. Spacer arms in affinity chromatography: use of hydrophilic arms to control or eliminate nonbiospecific adsorption effects. FEBS Lett. 1974 Jul 15;43(2):169–175. doi: 10.1016/0014-5793(74)80993-2. [DOI] [PubMed] [Google Scholar]
- Spratt B. G. Distinct penicillin binding proteins involved in the division, elongation, and shape of Escherichia coli K12. Proc Natl Acad Sci U S A. 1975 Aug;72(8):2999–3003. doi: 10.1073/pnas.72.8.2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spratt B. G., Jobanputra V. Mutants of Escherichia coli which lack a component of penicillin-binding protein 1 are viable. FEBS Lett. 1977 Jul 15;79(2):374–378. doi: 10.1016/0014-5793(77)80824-7. [DOI] [PubMed] [Google Scholar]
- Spratt B. G., Pardee A. B. Penicillin-binding proteins and cell shape in E. coli. Nature. 1975 Apr 10;254(5500):516–517. doi: 10.1038/254516a0. [DOI] [PubMed] [Google Scholar]
- Spratt B. G. Properties of the penicillin-binding proteins of Escherichia coli K12,. Eur J Biochem. 1977 Jan;72(2):341–352. doi: 10.1111/j.1432-1033.1977.tb11258.x. [DOI] [PubMed] [Google Scholar]
- Spratt B. G., Strominger J. L. Identification of the major penicillin-binding proteins of Escherichia coli as D-alanine carboxypeptidase IA. J Bacteriol. 1976 Jul;127(1):660–663. doi: 10.1128/jb.127.1.660-663.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strominger J. L., Willoughby E., Kamiryo T., Blumberg P. M., Yocum R. R. Penicillin-sensitive enzymes and penicillin-binding components in bacterial cells. Ann N Y Acad Sci. 1974 May 10;235(0):210–224. doi: 10.1111/j.1749-6632.1974.tb43267.x. [DOI] [PubMed] [Google Scholar]
- Suginaka H., Blumberg P. M., Strominger J. L. Multiple penicillin-binding components in Bacillus subtilis, Bacillus cereus, Staphylococcus aureus, and Escherichia coli. J Biol Chem. 1972 Sep 10;247(17):5279–5288. [PubMed] [Google Scholar]
- Suzuki H., Nishimura Y., Hirota Y. On the process of cellular division in Escherichia coli: a series of mutants of E. coli altered in the penicillin-binding proteins. Proc Natl Acad Sci U S A. 1978 Feb;75(2):664–668. doi: 10.1073/pnas.75.2.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamaki S., Nakajima S., Matsuhashi M. Thermosensitive mutation in Escherichia coli simultaneously causing defects in penicillin-binding protein-1Bs and in enzyme activity for peptidoglycan synthesis in vitro. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5472–5476. doi: 10.1073/pnas.74.12.5472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura T., Imae Y., Strominger J. L. Purification to homogeneity and properties of two D-alanine carboxypeptidases I From Escherichia coli. J Biol Chem. 1976 Jan 25;251(2):414–423. [PubMed] [Google Scholar]
- Waxman D. J., Strominger J. L. Cleavage of a COOH-terminal hydrophobic region from D-alanine carboxypeptidase, a penicillin-sensitive bacterial membrane enzyme. Characterization of active, water-soluble fragments. J Biol Chem. 1979 Jun 10;254(11):4863–4875. [PubMed] [Google Scholar]