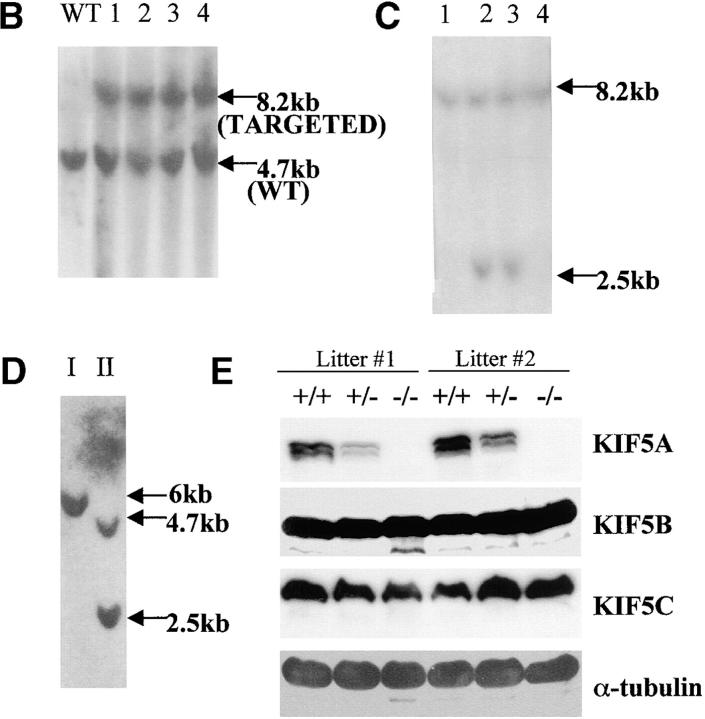
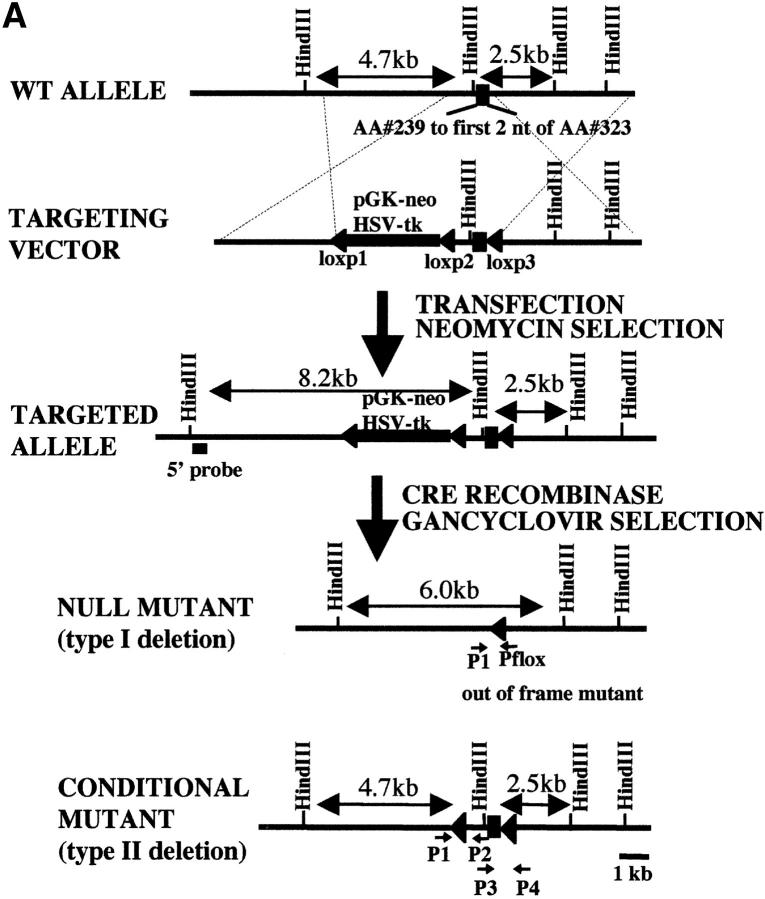
Figure 1.
Targeted disruption of the mouse KIF5A gene. (A) KIF5A gene targeting strategy. In the targeting vector, the pGK-neo and HSV-tk selection cassette was flanked by two loxP sites (loxP1 and loxP2), and the two exons to be deleted were flanked by loxP2 and loxP3. Two steps of transfection were performed to generate type I deletion (null mutant) and type II deletion ES cells. The first step was done by transfecting linearized targeting vector into ES cells followed by G418 selection; the second step was performed by transfecting a Cre plasmid into recombinant ES cells isolated from the first step, followed by Gancyclovir selection. (B and C) Southern blot analyses of HindIII-digested G418-resistant ES clones after transfecting targeting vector. (B) A 5′ external probe was used to identify the recombinant clones. A 4.7-kb wild-type band was detected, and an additional 8.2 kb (with the addition of a 3.5-kb selection cassette) was also detected in the recombinant clones. (C) The presence of three loxP sites was confirmed by a loxP probe. The correct recombinant clones should have all three loxP sites, both 8.2-kb and 2.5-kb bands should be detected by the probe (clones 2 and 3), whereas clones 1 and 4 only had the first two loxP sites. (D) Southern blot analysis of HindIII-digested Gancyclovir-resistant clones after Cre transfection. With the loxP probe, only a 6-kb band was detected in type I deletion (KIF5Anull) ES cells, whereas 4.7-kb and 2.5-kb bands were detected in type II (KIF5Aflox) ES cells. (E) No KIF5A protein was detected in KIF5A null mutant mice by Western blot analysis. Mouse brain homogenates were made from two litters of mice, and 100 μg of protein was loaded in each lane. Isoform-specific antibodies were used to probe KIF5A, KIF5B, and KIF5C; α-tubulin was used as a loading control.