Figure 8.
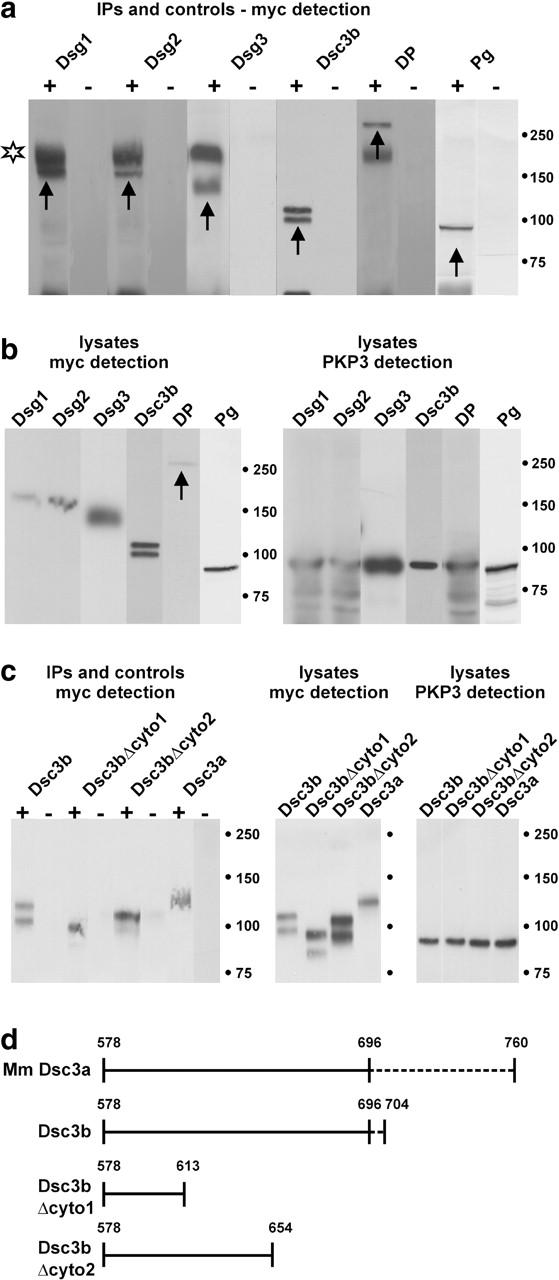
CoIP experiments using lysates of HEK293T cells cotransfected with plasmids of interest. A plasmid encoding full-length PKP3 (p1744) was cotransfected with plasmids encoding Dsg1-myc, Dsg2-myc, Dsg3-myc, Dsc3a-myc, Dsc3b-myc, Dsc3bΔcyto1-myc, Dsc3bΔcyto2-myc, DP-myc, or Pg-myc fusion proteins. (a) Using anti-PKP3 mAb 23E3/4, myc-tagged Dsg1, Dsg2, Dsg3, Dsc3b, DP, and Pg proteins (arrows) were coimmunoprecipitated with PKP3 (+ lanes). The nature of the Dsc3b doublet is unclear, but might represent incompletely processed protein in addition to mature protein. In the negative control lanes (lysates incubated with protein G Sepharose in the absence of mAb 23E3/4), no signal could be detected (− lanes). Incompletely reduced primary antibody was often detected (star). Identical exposure times were used for each set of +/− lanes. (b) Control Western blot detection of total cell lysates: Dsg1 (165 kD), Dsg2 (160 kD), Dsg3 (135 kD), Dsc3b (99 kD), DP (250 kD, arrow), and Pg (82 kD) in the left panel; PKP3 (87 kD) in the right panel. Mol wt markers (in kD) are indicated. (c) Using anti-PKP3 mAb 23E3/4, myc-tagged Dsc3b, two COOH-terminally truncated derivatives of Dsc3b and Dsc3a were coimmunoprecipitated with PKP3 (+ lanes in left panel). In the negative control lanes (lysates incubated with protein G Sepharose in the absence of mAb 23E3/4), no signal could be detected (− lanes in left panel). Expression of each of these fusion proteins was detected by Western blotting (right), using anti-Myc and anti-PKP3 antibodies. (d) Schematic representation of the cytoplasmic domains of mouse (Mm) Dsc3a, Dsc3b, and Dsc3b truncation mutants used in the CoIP experiments of (C). Dashed lines indicate isoform-specific domains. The fragment containing aa 578–696 is shared by Dsc3a and Dsc3b. Dsc3bΔcyto1 and Dsc3bΔcyto2 encompass, respectively, 36 and 77 membrane-proximal aa of both Dsc3a and -b.
