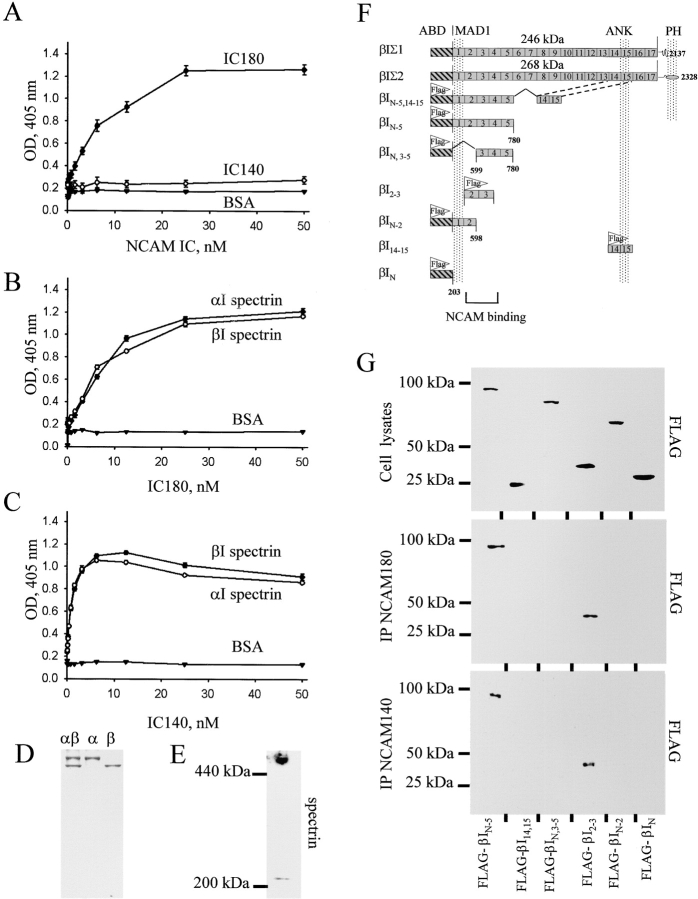
Figure 3.
The intracellular (IC) domains of NCAM180 and NCAM140 interact with the NH2-terminal region of spectrin. (A–C) Increasing concentrations of the IC domain of NCAM180 or NCAM140 were bound to plastic and assayed by ELISA for their ability to bind heterodimeric human erythrocyte spectrin (αIβI) or isolated αI and βI spectrin subunits. Binding to BSA served as a control. Mean values ± SEM from six independent experiments are shown. Note that heterodimeric spectrin bound well to IC180, whereas the isolated subunits bound to both IC180 and IC140. (D) Coomassie blue staining of the purified αIβI dimers and the isolated αI and βI spectrin subunits. (E) Isolated brain membranes assayed for βI spectrin by immunoblotting after PAGE under nondenaturing conditions. Note the low molecular weight band representing βI spectrin monomers. (F) Schematic alignment of βI spectrin fragments. For comparison, the βIΣ1 and βIΣ2 isoforms of βI spectrin are shown. These differ by alternative mRNA splicing at their COOH terminus, which contains the pleckstrin homology (PH) domain. ABD, actin-binding domain; MAD1, membrane association domain 1; ANK, ankyrin-binding domain. (G) Lysates of CHO cells transfected with NCAM180 or NCAM140 together with FLAG-labeled spectrin fragments were immunoblotted with FLAG antibodies to confirm approximately equal expression of each of the constructs (top). Lysates from NCAM180 (middle)– and NCAM140 (bottom)–transfected cells were immunoprecipitated (IP) with NCAM antibodies and immunoblotted with FLAG antibodies to detect which of the spectrin constructs would precipitate. Note that only βIN-5 and βI2–3 are present in the NCAM precipitates.