Figure 4.
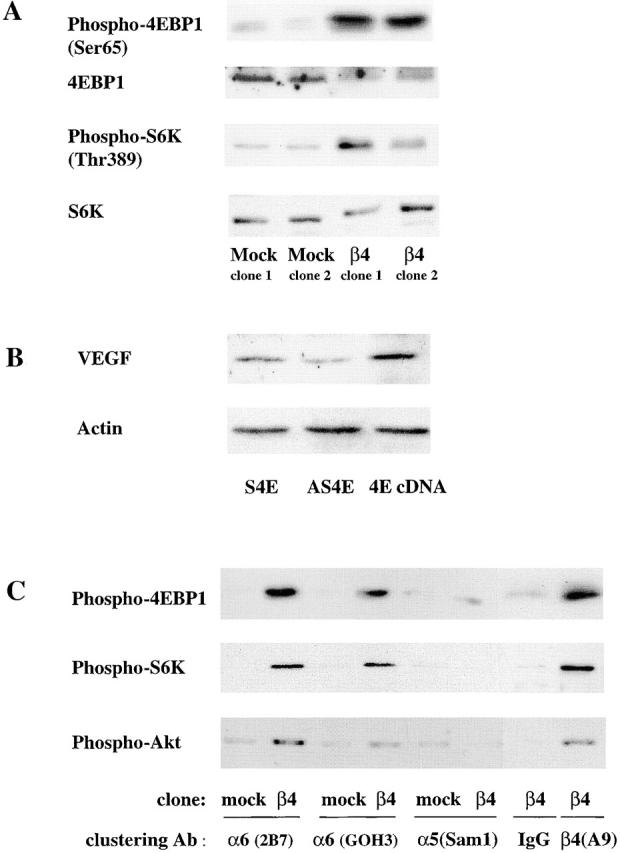
The α6β4 integrin stimulates the phosphorylation of Akt, 4E-BP1, and p70 S6K. (A) MDA-MB-435 parental cells, mock transfectants, and β4 transfectants were maintained in medium containing low serum (0.5% FBS) for 24 h. The phosphorylation status of 4E-BP1 on Ser 65 and S6K on Thr 389 was assessed in extracts from these cells using phosphospecific antibodies as described in the Materials and methods. In addition, the total amount of 4E-BP1 and p70S6K in these extracts was assessed by immunoblotting. (B) The MDA-MB-435/β4 cells were transiently transfected with either an eIF-4E sense (S) or antisense (AS) oligonucleotide, or a full-length eIF-4E cDNA (4E). Extracts of these cells containing equivalent amounts of protein were analyzed for their relative expression of VEGF and actin by immunoblotting. (C) MDA-MB-435 mock (clone 6D7) and β4 (clone 3A7) transfectants were maintained in low serum (0.5% FBS) medium for 24 h. These cells were detached with trypsin and incubated with integrin-specific antibodies (α6 integrin, 2B7; α6 integrin, GOH3; α5 integrin, Sam1; β4 integrin, A9) or IgG for 30 min as described in the legend to Fig. 2. The phosphorylation status of 4E-BP1 (Ser 65), S6K (Thr 389), and Akt (Ser 473) was assessed in extracts from these cells using phosphospecific antibodies. Similar results were observed in four independent experiments.
