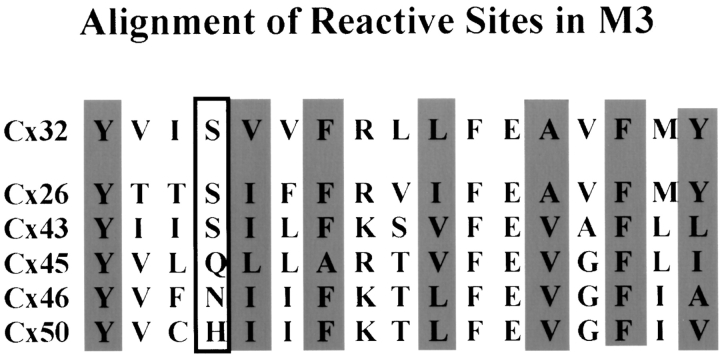
Figure 10.
Aligned amino acid sequences for the third transmembrane domain of Cx32 and several other connexins. Highlighted residues indicate pore-lining residues in Cx32 and their corresponding sites in other characterized connexins (open-boxed site only tested in the closed state). The periodicity in the NH2-terminal two-thirds of M3 suggests α-helix, but this breaks down at the COOH-terminal end. Many sites are strictly conserved, whereas others show relatively minor changes despite reports of significant differences in permeability between connexins. This suggests that subtle differences in residue length or branching may define the affinity of binding sites within the pore.