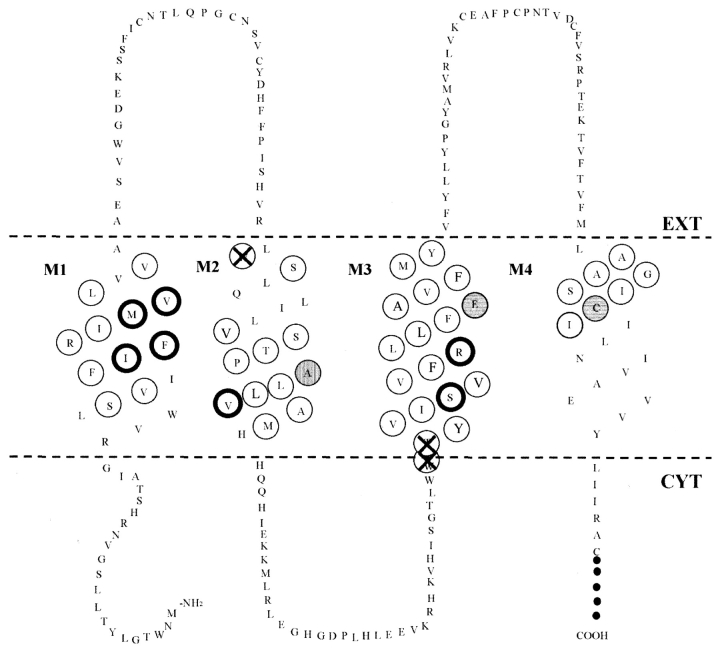
Figure 2.
Membrane topology of Cx32 showing all sites mutated to cysteine and analyzed in the current study. Cysteine substitutions were made at the indicated sites using overlapping PCR. Nonfunctional mutants are indicated with an “X.” At seven sites, cysteine substitution resulted in functional channels with a reverse-gating response to voltage (bold circles). Other sites where cysteine substitution altered channel properties (shaded circles) include A88 in M2, where cysteine substitution appears to induce hemichannel formation, and E146 in M3, where cysteine substitution results in a disulfide bond with C201 in M4 (Fig. 3, E and F). The topological plot is modified from Milks et al. (1988) to accommodate the empirical data demonstrating this proximity of E146 and C201. Extension of transmembrane spans beyond the margins shown here would be required for helices that are significantly tilted from the normal to the bilayer (helices C and D in Unger et al. [1999], corresponding to M3 and M4; Fig. 9).