Figure 8.
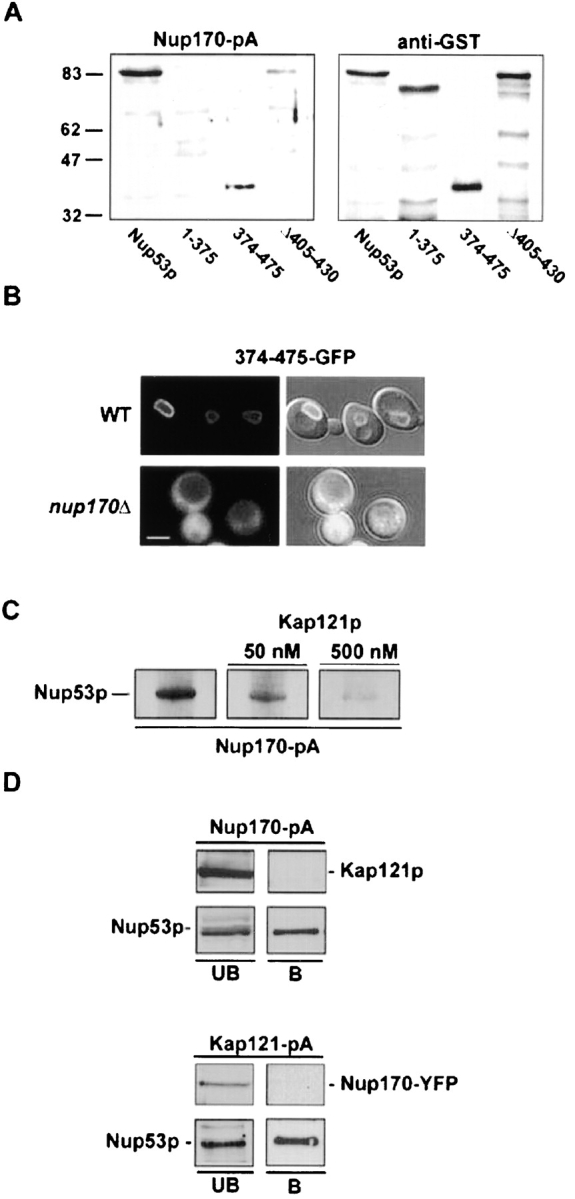
Nup170p and Kap121p interact with an overlapping region of Nup53p. (A) Nitrocellulose membranes containing SDS-PAGE–separated E. coli total cell lysates containing GST fusions of Nup53p and the indicated mutants were probed with either a cell lysate derived from a yeast strain expressing NUP170-pA (NP170pA) or an anti-GST antibody (α-GST). The positions of molecular mass markers of the indicated size in kilodaltons are shown on the left. (B) The subcellular distribution of the Nup170p binding domain of Nup53p was examined in WT (W303) and nup170Δ (NP170–11.1) strains using a 374–475-GFP fusion protein. Shown are confocal microscopy images acquired in both the fluorescent channel and a combination of the fluorescent and DIC images. (C) Equivalent amounts of GST–Nup53p in E. coli cell lysates were separated by SDS-PAGE, transferred to nitrocellulose and probed separately with an equal amount of a yeast total cell lysate containing Nup170-pA and supplemented with 0-, 50-, or 500-nM concentrations of recombinant Kap121p. The Nup170-pA fusion was detected using HRP-conjugated IgG antibodies and ECL. (D) Total cell lysates derived from yeast strains synthesizing Nup170-pA (NP170pA) or Kap121-pA (KP121pA/NP170YFP) were immunopurified on IgG–Sepharose columns. Shown are the unbound fraction (UB) and a fraction eluted from the beads with 1 M MgCl2 (B) analyzed by immunoblotting with α-Nup53p, α-GFP, or α-Kap121p antibodies to detect the indicated protein. Bar, 5 μm.
