Abstract
The evolution of mitogenic pathways has led to the parallel requirement for negative control mechanisms, which prevent aberrant growth and the development of cancer. Principally, such negative control mechanisms are represented by tumor suppressor genes, which normally act to constrain cell proliferation (Macleod, K. 2000. Curr. Opin. Genet. Dev. 10:81–93). Tuberous sclerosis complex (TSC) is an autosomal-dominant genetic disorder, characterized by mutations in either TSC1 or TSC2, whose gene products hamartin (TSC1) and tuberin (TSC2) constitute a putative tumor suppressor complex (TSC1-2; van Slegtenhorst, M., M. Nellist, B. Nagelkerken, J. Cheadle, R. Snell, A. van den Ouweland, A. Reuser, J. Sampson, D. Halley, and P. van der Sluijs. 1998. Hum. Mol. Genet. 7:1053–1057). Little is known with regard to the oncogenic target of TSC1-2, however recent genetic studies in Drosophila have shown that S6 kinase (S6K) is epistatically dominant to TSC1-2 (Tapon, N., N. Ito, B.J. Dickson, J.E. Treisman, and I.K. Hariharan. 2001. Cell. 105:345–355; Potter, C.J., H. Huang, and T. Xu. 2001. Cell. 105:357–368). Here we show that loss of TSC2 function in mammalian cells leads to constitutive S6K1 activation, whereas ectopic expression of TSC1-2 blocks this response. Although activation of wild-type S6K1 and cell proliferation in TSC2-deficient cells is dependent on the mammalian target of rapamycin (mTOR), by using an S6K1 variant (GST-ΔC-S6K1), which is uncoupled from mTOR signaling, we demonstrate that TSC1-2 does not inhibit S6K1 via mTOR. Instead, we show by using wortmannin and dominant interfering alleles of phosphatidylinositide-3-OH kinase (PI3K) that increased S6K1 activation is contingent upon the suppression of TSC2 function by PI3K in normal cells and is PI3K independent in TSC2-deficient cells.
Keywords: TSC; S6K1; 4E-BP1; PI3K; rapamycin
Introduction
Tuberous sclerosis complex (TSC)* is characterized by mutations in either TSC1 or TSC2 tumor suppressor genes, which appear to act together as a complex of the encoded proteins hamartin (TSC1) and tuberin (TSC2) (van Slegtenhorst et al., 1998). At least 1 in 6,000 newborns carries a constitutional TSC1 or TSC2 mutation and develops tumors in the brain, kidneys, skin, or other organs (Young and Povey, 1998). Although malignancy seldom develops, the effects of the disease can be devastating, including mental retardation, epilepsy, autism, pulmonary failure, kidney dysfunction, and disfigurement (Gomez et al., 1999). Because deficiency in either TSC1 or -2 leads to a very similar disease phenotype in humans with TSC (Jones et al., 1999), and hamartin and tuberin form a complex in both mammalian cells (van Slegtenhorst et al., 1998) and in Drosophila (Gao and Pan, 2001; Potter et al., 2001), an important common function of the TSC1-2 complex has been sought that could explain its tumor suppressor activity. Individually, hamartin contains a COOH-terminal ezrin-radixin-moesin–binding domain and has been implicated in signaling to the actin cytoskeleton (Lamb et al., 2000), whereas tuberin has an NH2-terminal leucine zipper necessary for hamartin binding (Hodges et al., 2001), a COOH-terminal domain similar to that found in GTPase-activating proteins (The European Chromosome 16 Tuberous Sclerosis Consortium, 1993), and may be involved in membrane trafficking (Kleymenova et al., 2001).
The effects of TSC deficiency that could provide clues to the functions of the TSC1-2 complex in mammalian tissues have been hampered by the embryonic lethality caused by homozygous TSC1 or -2 deficiency in knockout mice (Kobayashi et al., 1999, 2001; Onda et al., 1999; Kwiatkowski et al., 2002). However, recent studies in Drosophila have provided compelling evidence that the fly orthologues of TSC1 and -2 regulate cell size both in larval and adult tissues (Gao and Pan, 2001; Potter et al., 2001; Tapon et al., 2001). Additional effects on the cell cycle in Drosophila were also reported in these studies. These include an increase in cell number and shortening of the G1 phase during eye imaginal disc development (Tapon et al., 2001), as well as inappropriate cell proliferation of post-mitotic cells (Potter et al., 2001; Tapon et al., 2001). The effect of TSC deficiency on cell size has been particularly amenable to genetic epistasis analysis in the fly. This approach indicates that TSC1-2 functions in insulin/phosphatidylinositide-3-OH kinase (PI3K)–regulated signaling pathways, which control cell size upstream of Drosophila p70 S6 kinase (S6K) (Gao and Pan, 2001; Potter et al., 2001; Tapon et al., 2001). Three models were proposed to explain the observed genetic epistatic interactions between TSC1-2 and S6K (Potter et al., 2001): (1) TSC1-2 is a direct downstream target of protein kinase B (PKB), until recently a presumed upstream effector of mammalian target of rapamycin (mTOR) (Radimerski et al., 2002); (2) TSC1-2 acts on a parallel pathway that integrates the insulin signaling pathway at the level of S6K; or (3) TSC1-2 acts on a common downstream target of S6K. Here we provide evidence of a biochemical link between TSC1-2 and S6K1, and establish that mTOR and PI3K signaling act separately to activate S6K1, the latter by repressing TSC1-2.
Results and discussion
TSC1-2 negatively regulates the phosphorylation and activity of S6K1
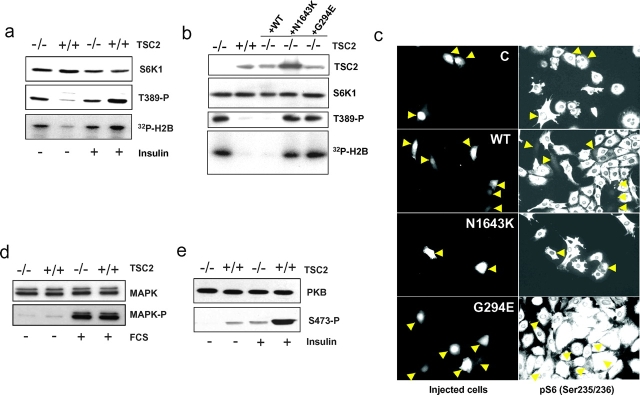
To address the relationship between TSC1-2 and S6K in mammals, advantage was taken of mouse embryo fibroblasts (MEFs) derived from mice deficient for TSC2 and a second tumor suppressor gene, p53. Removal of p53 was required for TSC2−/− MEFs to proliferate in culture. Analysis of S6K1 T389 phosphorylation and kinase activity in TSC2−/− MEFs shows that both responses are constitutive and refractile to either mitogen withdrawal or stimulation by insulin (Fig. 1 a). In control p53−/− MEFs, both responses are basal in the absence of mitogens and acutely stimulated by the addition of insulin (Fig. 1 a). Stable transfection or microinjection of wild-type human TSC2, but not either of two TSC disease–associated point mutants, including a recently described TSC2 mutant unable to bind TSC1 (Hodges et al., 2001), restores regulated S6K1 T389 phosphorylation, kinase activity, and S6 S235/S236 phosphorylation (Fig. 1, b and c). These findings demonstrate that these effects are not attributable to the absence of p53, and are consistent with mutations thought to be the cause of TSC. To determine if the effects of loss of TSC2 function were selective for the S6K1 signaling pathway, the activities of MAPK and PKB were also analyzed in the same cell lines by using phosphospecific antibodies. The results show that serum stimulation of MAPK phosphorylation was unaffected in TSC2−/− MEFs as compared with control MEFs (Fig. 1 d). However, insulin-induced PKB S473 phosphorylation was strongly suppressed in TSC2 −/− MEFs (Fig. 1 e) as was PKB activity (see below). The effect of loss of TSC2 function on PKB activation may reflect a recently described negative feedback loop from mTOR/S6K to the PI3K signaling pathway (Haruta et al., 2000), where rapamycin treatment leads to increased signaling in the PI3K signaling pathway. Thus, in the case of loss of TSC2 function, constitutive S6K1 signaling may be expected to act in the opposite manner to inhibit signaling in the PI3K pathway, such that PKB activity is largely suppressed in TSC2-deficient cells. Taken together, the results support a model whereby TSC1-2 operates as an upstream negative effector of S6K1, rather than affecting a common downstream target.
Figure 1.
The effects of TSC2 deficiency on the insulin signal transduction pathway. (a) Serum-deprived TSC2−/− and TSC2+/+ MEFs were extracted directly or after stimulation with 200 nM insulin. S6K1 levels, T389 phosphorylation, and S6K1 activity were assayed as described previously (Dennis et al., 2001). (b) TSC2−/− and TSC2+/+ MEFs and TSC−/− MEFs stably transfected (left) with either wild-type (+WT) TSC2 or TSC2 N1643K (+N1643K) or G294E (+G294E) mutants were extracted after serum deprivation for 24 h. TSC2 levels were measured by Western blot analysis. S6K1 expression levels, T389 phosphorylation, and kinase activity were as in panel a. (c) TSC2−/− MEFs were microinjected with empty pCMVTag vector (C) or the same vector encoding either wild-type TSC2 (WT) or N1643K or G294E mutants, and inhibition of S6K was detected using an antibody against phospho-S6 (Ser235/236). Arrows indicate microinjected cells. (d and e) Expression levels and phosphorylation of PKB and MAPK were measured by Western blot analysis after serum starvation or after stimulation for 30 min.
The translational inhibitor 4E-BP1 is regulated by TSC1-2
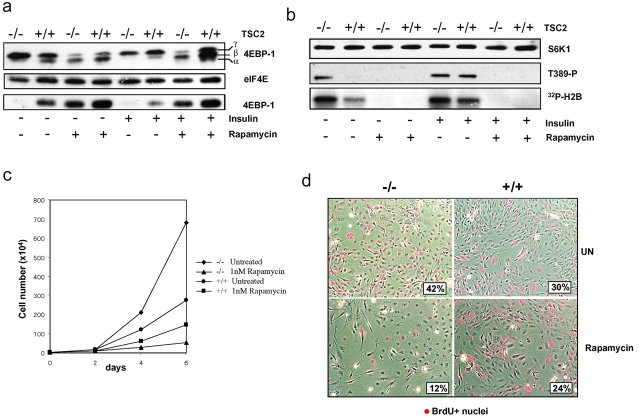
Phosphorylation of T389 of S6K1 is mediated by mTOR (Burnett et al., 1998), as is the phosphorylation of the translation inhibitor, initiation factor 4E binding protein, 4E-BP1 (Brunn et al., 1997). Analysis of 4E-BP1 in serum-deprived TSC2−/− MEFs indicates that 4E-BP1 is highly phosphorylated, as judged by its qualitatively reduced electrophoretic mobility, whereas the less highly phosphorylated derivatives of 4E-BP1 are detectable in control MEFs (Fig. 2 a). Consistent with this interpretation, 4E-BP1 is not associated with translational initiation complexes containing 7-methyl cap–bound eIF4E from extracts of TSC2−/− MEFs, but is clearly present in extracts from control MEFs (Fig. 2 a). Insulin stimulation has no further effect on either phosphorylation or activity of 4E-BP1 in TSC2−/− MEFs, but leads to increased phosphorylation of 4E-BP1 and its release from eIF4E in control MEFs (Fig. 2 a). In contrast, rapamycin treatment of either cell type in the absence or presence of insulin leads to 4E-BP1 dephosphorylation and its reassociation with eIF4E (Fig. 2 a). Similarly, rapamycin treatment induces S6K1 T389 dephosphorylation and kinase inactivation in both TSC2-expressing or -deficient cells treated with and without insulin (Fig. 2 b).
Figure 2.
Inhibition of 4E-BP1, S6K1, and proliferation by rapamycin in TSC2 −/− and TSC2 +/+ MEFs. (a) Serum-starved MEFs were extracted directly or after stimulation with 200 nM insulin in the absence or presence of 20 nM rapamycin. Phosphorylation of 4E-BP1, 4E-BP1 association with 7-methyl cap–bound eIF4E, and eIF4E levels were measured by Western blot analysis as previously described (von Manteuffel et al., 1997). (b) S6K1 levels, T389 phosphorylation, and kinase activity were as in Fig. 1 a. (c) Proliferation of TSC2−/− and TSC2+/+ MEFs was determined by culturing MEFs for the indicated times in the absence or presence of 1 nM rapamycin. Each value represents the average number of cells from three independent experiments, with the cell number from each culture determined by counting three independent dishes. Filled diamonds, TSC2−/−, untreated; filled triangles, TSC2−/− with 1 nM rapamycin; filled circles, TSC2+/+, untreated; filled squares, TSC2+/+ with 1 nM rapamycin. (d) Dual phase contrast and anti-BrdU–labeled nuclei (red) images of TSC2+/+ and TSC2−/− MEFs left untreated for 4 d (UN, top) or in the presence of 1 nM rapamycin (Rapamycin, bottom). Inset shows percentage of BrdU-positive nuclei.
In parallel, we examined the effect of rapamycin on the proliferation of TSC2−/− and control MEFs. The results show that TSC2−/− MEFs grow more rapidly than control MEFs, but that they are strikingly more sensitive to inhibition of proliferation by rapamycin (Fig. 2 c). Similar results were obtained by examining the proportion of cycling cells by BrdU labeling after treatment with low concentrations of rapamycin (Fig. 2 d). These findings indicate that TSC2 negatively regulates the activity and phosphorylation of both S6K1 and 4E-BP1 to restrict proliferation in normal cells.
TSC1-2 acts independently of mTOR on GST-ΔC-S6K1
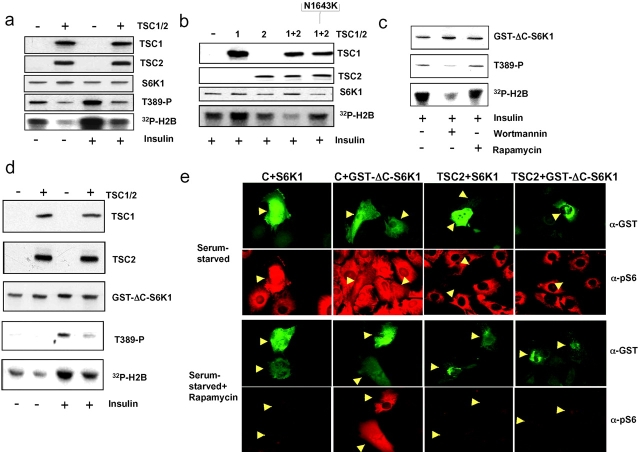
That both S6K1 and 4E-BP1 are constitutively phosphorylated in TSC2−/− MEFs and sensitive to rapamycin, argues that TSC1-2 may act directly to inhibit mTOR function. However, these findings do not exclude the possibility that TSC1-2 functions either in a parallel pathway or downstream of mTOR to regulate the activities of both S6K1 and 4EBP-1. If TSC1-2 acts as a negative regulator of S6K1 through inhibiting mTOR function, S6K1 inactivation by TSC1-2 overexpression should occur in an mTOR-dependent manner. To test this, we first developed an assay in which epitope-tagged variants of human TSC1 and TSC2 were ectopically expressed in Cos cells together with a wild-type S6K1 reporter. Under these conditions, expression of TSC1-2 reduced basal and insulin-induced S6K1 reporter T389 phosphorylation and kinase activation (Fig. 3 a). In contrast, in a separate experiment, cotransfection of either TSC1 or TSC2 alone or the human TSC2 N1643K mutant (Fig. 1 b) with TSC1 had no significant effect on insulin-induced S6K1 activation (Fig. 3 b). To determine whether inhibition of S6K1 by TSC1-2 was mTOR dependent, advantage was taken of a recently described variant of S6K1, GST–ΔC-S6K1, in which the COOH-terminal autoinhibitory domain has been deleted and GST fused to the NH2 terminus (Dennis et al., 2001). Consistent with earlier findings, insulin-induced GST–ΔC-S6K1 T389 phosphorylation and activation are sensitive to the PI3K inhibitor wortmannin, but resistant to rapamycin, and therefore uncoupled from mTOR (Fig. 3 c). Unexpectedly, using the Cos cell overexpression assay, coexpression of TSC1 and wild-type TSC2 also suppressed insulin-induced GST–ΔC-S6K1 reporter T389 phosphorylation and activation (Fig. 3 d), and thus phenocopied wortmannin but not rapamycin treatment. To confirm these results, we used an alternative assay in which TSC1-2 regulates S6K1. Because reintroduction of wild-type TSC2 by microinjection into TSC2−/− MEFs restores serum-regulated S6K1 activity, presumably through forming complexes with endogenous TSC1 (Fig. 1, b and c), we reasoned that it may similarly regulate the activity of either ectopically expressed S6K1 or GST–ΔC-S6K1. However, given the findings above we would expect GST–ΔC-S6K1 to be resistant to the effects of rapamycin. Indeed, introduction of TSC2 together with either S6K1 or GST–ΔC-S6K1 led to kinase inhibition in serum-derived TSC2−/− MEFs in the presence or absence of rapamycin as assessed by phospho-S6 staining (Fig. 3 e). In contrast, only GST–ΔC-S6K1 was active when introduced with control vector in the presence of rapamycin, confirming that this S6K1 variant is indeed mTOR independent, but still regulated by TSC2. Taken together, these findings argue that TSC1-2 inhibition of S6K1 is unlikely to be via direct regulation of mTOR.
Figure 3.
The inhibitory effects of TSC1-2 on wild-type S6K1 and GST-ΔC-S6K1. (a) Cos cells were cotransfected with S6K1 and Flag-tagged TSC1 and TSC2 expression constructs, and after serum deprivation, left untreated or stimulated with 200 nM insulin for 30 min. (b) Cos cells were cotransfected with S6K1 and TSC1 alone, TSC2 alone, TSC1 and wild-type TSC2 together, or TSC1 and N1643K TSC2 mutant together. Cells were extracted after stimulation with 200 nM insulin. Expression levels of S6K1 as well as T389 phosphorylation and kinase activity were determined as in Fig. 1. TSC1 and TSC2 were detected with a Flag epitope antibody. (c) Cos cells transfected with GST–ΔC-S6K1 (Dennis et al., 2001) were stimulated with 200 nM insulin alone or in the presence of either 100 nM wortmannin or 20 nM rapamycin. (d) Cos cells were cotransfected with GST–ΔC-S6K1 together with TSC1 and -2 and stimulated with insulin (Fig. 3 a). Expression levels of TSC1 and TSC2, S6K1 phosphorylation, and activity of S6K were as in panel b. (e) TSC2−/− MEFs microinjected with pCMVTag empty control vector (C) or pCMVTag vector encoding wild-type TSC2 (TSC2) in combination with expression vectors encoding GST-tagged wild-type S6K1 (+S6K1) or GST–ΔC-S6K1 after 12 h serum starvation (Serum-starved) alone or followed by treatment with 10 nM rapamycin (Serum starved + Rapamycin). Images are of cells expressing GST-tagged kinases (α-GST, green) and S6K1 activity measured using an antibody to phospho-S6 (α-pS6, red).
PI3K signaling antagonizes S6K1 inhibition by TSC1-2 in normal cells
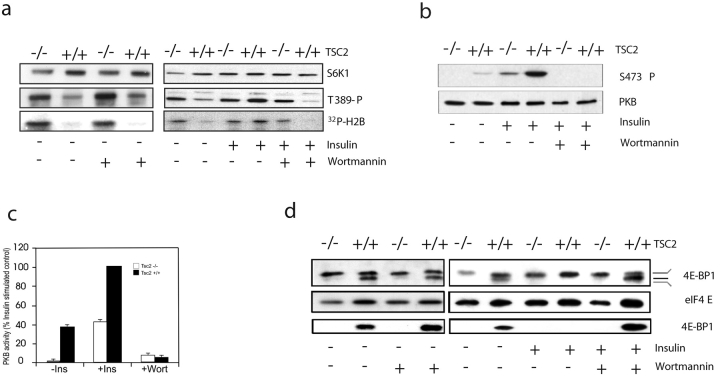
The results above show that insulin stimulation of the rapamycin-resistant variant of S6K1 is inhibited to approximately the same extent by either wortmannin or TSC1-2 overexpression (Fig. 3, b and c), raising the possibility that TSC1-2 inhibition of S6K1 is suppressed by insulin-induced PI3K activation. Consistent with this model, in most mammalian cell types, S6K1 activation is blocked by either the PI3K inhibitor wortmannin or by dominant interfering alleles of PI3K (Reif et al., 1997). To test whether TSC1-2 repression is mediated by PI3K, both TSC2−/− and control MEFs were treated with wortmannin in the absence or presence of insulin (Fig. 4 a). In the absence of insulin stimulation, increased T389 phosphorylation and S6K1 activity are unaffected by wortmannin treatment in TSC2−/− MEFs (Fig. 4). Likewise, wortmannin has little affect on either response in these same cells in the presence of insulin, but both responses are blocked by the drug in control cells (Fig. 4 a), implying that the role of PI3K in S6K activation in normal cells may be to repress TSC1-2. Consistent with wortmannin inhibiting PI3K signaling, and the specificity of TSC1-2 for S6K1, treatment of either cell type with the drug abolished PKB S473 phosphorylation (Fig. 4 b) and kinase activity using the synthetic peptide Crosstide (Cross et al., 1995) as a substrate in an immune complex assay (Fig. 4 c).
Figure 4.
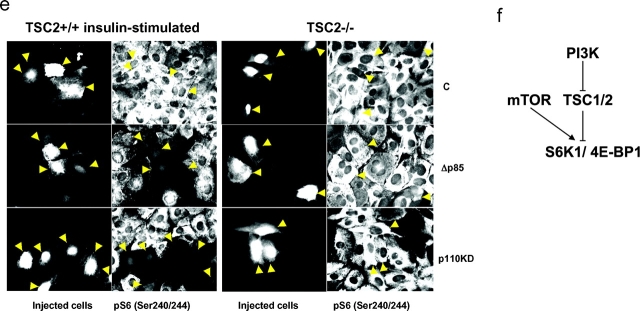
The repression of S6K1 and 4E-BP1 by TSC1-2 is mediated by PI3K. (a) TSC2−/− and TSC2+/+ MEFs were extracted directly or after stimulation in the absence or presence of 100 nM wortmannin. Expression of S6K1, T389 phosphorylation, and S6K1 activity were measured as in Fig. 1. (b) Expression and phosphorylation of PKB were determined as in Fig. 1 c. (c) Activity measurement of PKB was analyzed as described previously (Pullen et al., 1998). (d) 4E-BP1 phosphorylation and levels of eIF4E were as described in Fig. 2 b. (e) TSC2+/+ and TSC2−/− MEFs were microinjected with expression plasmids encoding either an empty pCMVTag vector (C; top), a p85 deletion mutant (Δp85; middle), or a kinase-inactive PI3K (p110KD; bottom). S6 phosphorylation was detected using an antibody against phospho-S6. (f) Model showing that PI3K mediates insulin induction of S6K1 and 4E-BP1 through repression of TSC1-2.
These findings raised the possibility that in normal cells, TSC1-2 might also regulate 4E-BP1 phosphorylation in a PI3K-dependent manner. To test this, we examined the effect of wortmannin on the phosphorylation status of 4E-BP1 and its association with initiation factor eIF4E in both TSC2-expressing or -deficient MEFs in the absence and presence of insulin. The results show that wortmannin has no effect on the maximally phosphorylated state of 4E-BP1 in either untreated or insulin-treated TSC2−/− MEFs, or on its interaction with initiation factor eIF4E (Fig. 4 d). In contrast, insulin-induced 4E-BP1 phosphorylation and its release from initiation factor 4E are abolished by wortmannin treatment in control MEFs (Fig. 4 d). Consistent with these findings, microinjection of either kinase-inactive PI3K or a deletion mutant of the PI3K p85 subunit blocks S6 S235/S236 phosphorylation in control MEFs, but not in TSC2−/− MEFs (Fig. 4 e). These results indicate that constitutive S6K1 activity in TSC2-deficient cells is not due to deregulated PI3K signaling, and support a model whereby in normal cells PI3K mediates insulin-induced S6K1 and 4E-BP1 phosphorylation through repressing TSC1-2 function (Fig. 4 f).
TSC1-2 mechanism of action in regulating S6K1 and 4E-BP1
Mitogen-induced S6K1 activation is mediated by the phosphorylation of residues residing in its linker and conserved catalytic domains in a hierarchical manner via mTOR and the phosphoinositide-dependent protein kinase 1, respectively (Isotani et al., 1999). Despite prevailing models that both kinases are downstream effectors of PI3K with respect to S6K1 activation (Blume-Jensen and Hunter, 2001), recent studies indicate that this is not the case for either kinase (Pullen et al., 1998; Dennis et al., 2001). Instead, both kinases appear constitutively active regardless of mitogenic ligand stimulation. Our findings demonstrate that in normal cells the PI3K input to S6K1 is the suppression of TSC1-2, which we hypothesize may normally act as a positive effector of a S6K1 phosphatase. Alternatively, given the potential role of TSC2 in trafficking to the plasma membrane (Kleymenova et al., 2001), TSC1-2 complexes may act to partition S6K1 in a compartment of the cell where access by activating kinases is limited.
However, it also should be noted that in a few cell types and in Drosophila (Radimerski et al., 2002), S6K1 activation can be PI3K independent, suggesting other mechanisms may exist to regulate TSC1-2 function. During the completion of these studies, others reported that S6K1 activity was elevated in TSC1-deficient cells and blocked by rapamycin and LY294002 (Kwiatkowski et al., 2002), a PI3K inhibitor (Vlahos et al., 1994). This led to the conclusion that PI3K induces S6K1 activation through mTOR and that mTOR is the target of TSC1-2 suppression. However, at the concentrations employed, LY294002 has been demonstrated to be a potent inhibitor of both PI3K and mTOR (Brunn et al., 1996). Thus, the fact that LY294002 inhibits phosphorylation of S6K1 in TSC1-deficient cells is most likely explained by inhibitory affects of the drug on mTOR rather than PI3K. Such an explanation would clarify why we observe little to no inhibitory affect of wortmannin or microinjection of either a kinase-inactive PI3K or a deletion mutant of the p85 subunit on S6K1 activity.
Our findings indicate that in normal cells, signaling via PI3K antagonizes the TSC1-2 complex and its ability to repress S6K1. During the review process, three other reports have appeared that suggest a mechanism of the PI3K-regulated TSC1-2 inactivation described here. Activation of the PI3K-regulated serine-threonine kinase Akt/PKB has been shown in these studies to lead to phosphorylation of TSC2 (Inoki et al., 2002; Manning et al., 2002; Potter et al., 2002), although the suggested mechanism of the resulting inhibition of the TSC1-2 complex varies between studies. Interestingly, these new reports place Akt/PKB upstream of S6K1, despite previous data to the contrary (Dufner et al., 1999, Radimerski et al., 2002). Further studies are clearly necessary to establish whether inactivation of TSC1-2 by the activated alleles of Akt/PKB commonly used in these types of studies is equivalent to physiological ligand-induced activation of PI3K signaling for S6K1 regulation. More importantly, it will be critical to establish whether such a mode of inactivation of the TSC1-2 complex by phosphorylation in normal cells is equivalent to the mode of inactivation of TSC1-2 by pathogenic TSC2 mutations. Finally, in one of these reports (Inoki et al., 2002), use of different rapamycin-resistant S6K1 constructs has allowed the authors to reach opposite conclusions from ours regarding whether TSC1-2 regulates S6K1 by regulating mTOR activity, or acts directly on S6K1. With the emerging importance of the mTOR–S6K–4E-BP signaling pathway in cancer (Gingras et al., 2001), it will be critical to resolve this issue and establish the molecular mechanisms by which TSC1-2 acts as a tumor suppressor in this pathway. Importantly, given the selective effects of rapamycin on the proliferation of TSC2−/− MEFs described here and its efficacious use in the treatment of solid tumors in phase 1 clinical trials (Hidalgo and Rowinsky, 2000), the use of this drug could be explored for the treatment of TSC and TSC-associated maladies.
Materials and methods
MEF cell lines
Mouse embryonic fibroblast lines from Tsc2−/−;p53−/− and Tsc2+/+;p53−/− embryos will be described elsewhere (provided by H. Onda and D. Kwiatkowski, Brigham and Women's Hospital, Boston, MA). In brief, E10–10.5 embryos were collected from Tsc2+/−;p53−/− intercrosses. The stage of embryos were estimated by counting days after vaginal plug was observed in female (considered to be E0.5) and confirmed by developmental stage of limb bud at the time of harvesting. Embryo viability was determined by cardiac contraction, however, in some cases nonviable “embryoid masses” are also used. Each embryo was triturated in DME, and then plated in DME supplemented with 10% FBS, 50 μg/ml penicillin, and 50 μg/ml streptomycin in 5% CO2. MEF cultures from each embryo were expanded and the genotype of each culture was initially determined by PCR of DNA isolated from extraembryonic membrane and further confirmed by Western blotting for tuberin expression in the cultured cells. All experiments were performed at early passages (<20). When possible, all experiments were performed on paired littermates. For measurement of proliferation, MEFs were seeded at 0.5 × 104 per 9-cm dish and cells were counted every second day for 6 d with rapamycin added to 1 μM every 2 d. For BrdU labeling, cells were seeded at the same density on 24-mm glass coverslips and 24 h later treated with 1 μM rapamycin for 4 d, before labeling with 5 μg/ml BrdU (Sigma-Aldrich) for 8 h. Cells were subsequently fixed and stained with a rat anti-BrdU monoclonal antibody and Hoechst to visualize cell nuclei. To count BrdU-positive cells, the total number of nuclei and BrdU-positive nuclei were counted in each of three 10× objective fields for each MEF line and condition and numbers averaged to give a percentage. Dual phase contrast and BrdU-positive images were obtained from 10× fields using Axiovision and Adobe Photoshop® software.
Transfection and retroviral reintroduction of TSC2 into TSC2− /− MEFs
For transient transfection of Cos cells, cells were seeded in 6-well plates at 2 × 105 per well. Transfection was performed using Fugene 6 from Roche. 24 h after transfection, cells were serum starved for 48 h and extracted directly or after stimulation with 200 nM insulin, with or without the addition of 20 nM rapamycin or 100 nM wortmannin. For retroviral transfection, the complete coding regions of wild-type TSC2 and the TSC N1643K and G294E mutants were excised as BamH1 fragments from pCMVTag2 TSC2 vectors and cloned into a modified retroviral vector based on pRevTRE (CLONTECH Laboratories, Inc.), in which the TRE-minimal CMV promoter was replaced with the minimal tk promoter from pBLCAT2. BOSC cells were used to package retroviruses and retroviral supernatants used to infect TSC2−/−/p53−/− MEFs. Retrovirally transduced populations were selected with hygromycin and 3T3 cell lines generated by limit dilution cloning.
Microinjection
TSC2+/+ or TSC2−/− MEFs on coverslips were nuclear microinjected with 0.1 mg/ml plasmids together with 5 mg/ml biotin–dextran (Molecular Probes) as an injection marker. For Fig. 1 c, TSC−/− MEFs were microinjected with 0.2 mg/ml empty pCMVTag vector or the same vector expressing wild-type TSC2 or TSC2 mutants. For Fig. 2 e, cells were injected with 0.5 mg/ml pCMVTag empty vector or pCMVTag wild-type TSC2 together with 0.1 mg/ml pRK5-GSTS6K1 or 0.3 mg/ml pRK5-GST–ΔC-S6K1. In both Fig. 1 c and Fig. 3 e, cells were serum starved after microinjection for 12 h and left untreated or treated with 10 nM rapamycin for a further 1 h before fixation. Expression of S6K1 was detected using a monoclonal antibody to GST (Santa Cruz Biotechnology, Inc.) and goat anti–mouse FITC. For Fig. 4 e, empty pCMVTag vector or expression plasmids encoding PI3K deltap85 (pcDNA3) or kinase-dead p110 (pEFBos; both from Dr. Julian Downward) were injected at 0.1 mg/ml. After serum starvation for 12 h, TSC2−/− were left unstimulated whereas TSC2+/+ cells were stimulated with 200 nM insulin for 1 h before fixation. All cells were fixed for 15 min with 4% paraformaldehyde in PBS, permeabilized for 10 min with PBS/0.4% Triton X-100, blocked for 15 min with PBS/1% BSA, and stained with an antibody to phospho-S6 (Ser235/236; Cell Signaling) followed by goat anti–rabbit TRITC (Molecular Probes). Injected cells were detected with Alexa®350-conjugated Streptavidin (Molecular Probes). Images were acquired on a ZEISS Axioplan 2 imaging microscope on 20× or 40× objectives with Axiovision software and processed either as greyscale or dual color TIFF images in Adobe Photoshop®.
Acknowledgments
The authors thank L. Harrington and S. Wigfield for helpful discussions. We thank F. Zilbermann for his expert technical assistance and Alessandro Di Cara for help in making figures. We also thank W. Filipowicz, W. Krek, and U. Mueller for their critical reading of the manuscript. We are indebted to D. Kwiatkowski for MEF cultures and E. Sahai (Institute of Cancer Research, London, UK) for construction of the retroviral vector and discussion. We also thank N. Sonenberg (McGill University, Montreal, Canada) for the 4E-BP1 antibody and T. Kobayashi (Cancer Institute, Tokyo, Japan) for Flag epitope–tagged TSC1 and TSC2.
These studies were supported by grants from Cancer Research UK, the Tuberous Sclerosis Association (UK), and the LAM Foundation (J. Hardkamp, W. Roworth, and R. Lamb) and a grant from the Swiss Cancer League to A. Jaeschke, T. Nobukuni, and G. Thomas. M. Saitoh was supported by a fellowship from the European Molecular Biology Organization, and G. Thomas was supported by the Novartis Research Foundation.
J. Hardkamp and M. Saitoh contributed equally to this work.
Footnotes
Abbreviations used in this paper: MEF, mouse embryo fibroblast; mTOR, mammalian target of rapamycin; PI3K, phosphatidylinositide-3-OH kinase; PKB, protein kinase B; S6K, S6 kinase; TSC, tuberous sclerosis complex.
References
- Blume-Jensen, P., and T. Hunter. 2001. Oncogenic kinase signalling. Nature. 411:355–365. [DOI] [PubMed] [Google Scholar]
- Brunn, G.J., J. Williams, C. Sabers, G. Wiederrecht, J.C. Lawrence, Jr., and R.T. Abraham. 1996. Direct inhibition of the signaling functions of the mammalian target of rapamycin by the phosphoinositide 3-kinase inhibitors, wortmannin and LY294002. EMBO J. 15:5256–5267. [PMC free article] [PubMed] [Google Scholar]
- Brunn, G.J., C.C. Hudson, A. Sekulic, J.M. Williams, H. Hosoi, P.J. Houghton, J.C. Lawrence, Jr., and R.T. Abraham. 1997. Phosphorylation of the translational repressor PHAS-I by the mammalian target of rapamycin. Science. 277:99–101. [DOI] [PubMed] [Google Scholar]
- Burnett, P.E., R.K. Barrow, N.A. Cohen, S.H. Snyder, and D.M. Sabatini. 1998. RAFT1 phosphorylation of the translational regulators p70 S6 kinase and 4E-BP1. Proc. Natl. Acad. Sci. USA. 95:1432–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross, D.A.E., D.R. Alessi, P. Cohen, M. Andjelkovic, and B.A. Hemmings. 1995. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature. 378:785–789. [DOI] [PubMed] [Google Scholar]
- Dennis, P.B., A. Jaeschke, M. Saitoh, B. Fowler, S.C. Kozma, and G. Thomas. 2001. Mammalian TOR: a homeostatic ATP sensor. Science. 294:1102–1105. [DOI] [PubMed] [Google Scholar]
- Dufner, A., M. Andjelkovic, B.M. Burgering, B.A. Hemmings, and G. Thomas. 1999. Protein kinase B localization and activation differentially affect S6 kinase 1 activity and eukaryotic translation initiation factor 4E-binding protein 1 phosphorylation. Mol. Cell. Biol. 19:4525–4534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The European Chromosome 16 Tuberous Sclerosis Consortium. 1993. Identification and characterization of the tuberous sclerosis gene on chromosome 16. Cell. 75:1305–1315. [DOI] [PubMed]
- Gao, X., and D. Pan. 2001. TSC1 and TSC2 tumor suppressors antagonize insulin signalling in cell growth. Genes Dev. 15:1383–1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gingras, A.C., B. Raught, and N. Sonenberg. 2001. Regulation of translation initiation by FRAP/mTOR. Genes Dev. 15:807–826. [DOI] [PubMed] [Google Scholar]
- Gomez, M.R., J.R. Sampson, and V.H. Whittemore. 1999. Tuberous Sclerosis. Third edition. Oxford University Press, New York. 340 pp.
- Haruta, T., T. Uno, J. Kawahara, A. Takano, K. Egawa, P.M. Sharma, J.M. Olefsky, and M. Kobayashi. 2000. A rapamycin-sensitive pathway down-regulates insulin signaling via phosphorylation and proteasomal degradation of insulin receptor substrate-1. Mol. Endocrinol. 14:783–794. [DOI] [PubMed] [Google Scholar]
- Hidalgo, M., and E.K. Rowinsky. 2000. The rapamycin-sensitive signal transduction pathway as a target for cancer therapy. Oncogene. 19:6680–6686. [DOI] [PubMed] [Google Scholar]
- Hodges, A.K., S. Li, J. Maynard, L. Parry, R. Braverman, J.P. Cheadle, J.E. DeClue, and J.R. Sampson. 2001. Pathological mutations in TSC1 and TSC2 disrupt the interaction between hamartin and tuberin. Hum. Mol. Genet. 10:2899–2905. [DOI] [PubMed] [Google Scholar]
- Inoki, K., Y. Li, T. Zhu, J. Wu, and K.-L. Guan. 2002. TSC2 is phosphorylated and inhibited by Akt and suppresses mTOR signalling. Nat. Cell Biol. 4:648–657. [DOI] [PubMed] [Google Scholar]
- Isotani, S., K. Hara, C. Tokunaga, H. Inoue, J. Avruch, and K. Yonezawa. 1999. Immunopurified mammalian target of rapamycin phosphorylates and activates p70 S6 kinase α in vitro. J. Biol. Chem. 274:34493–34498. [DOI] [PubMed] [Google Scholar]
- Jones, A.C., M.M. Shyamsundar, M.W. Thomas, J. Maynard, S. Idziaszczyk, S. Tomkins, J.R. Sampson, and J.P. Cheadle. 1999. Comprehensive mutation analysis of TSC1 and TSC2-and phenotypic correlations in 150 families with tuberous sclerosis. Am. J. Hum. Genet. 64:1305–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleymenova, E., O. Ibraghimov-Beskrovnaya, H. Kugoh, J. Everitt, H. Xu, K. Kiguchi, G. Landes, P. Harris, and C. Walker. 2001. Tuberin-dependent membrane localization of polycystin-1: a functional link between polycystic kidney disease and the TSC2 tumor suppressor gene. Mol. Cell. 7:823–832. [DOI] [PubMed] [Google Scholar]
- Kobayashi, T., O. Minowa, J. Kuno, H. Mitani, O. Hino, and T. Noda. 1999. Renal carcinogenesis, hepatic hemangiomatosis, and embryonic lethality caused by a germ-line TSC2 mutation in mice. Cancer Res. 59:1206–1211. [PubMed] [Google Scholar]
- Kobayashi, T., O. Minowa, Y. Sugitani, S. Takai, H. Mitani, E. Kobayashi, T. Noda, and O. Hino. 2001. A germ-line TSC1 mutation causes tumor development and embryonic lethality that are similar. but not identical, to those caused by TSC2 mutation in mice. Proc. Natl. Acad. Sci. USA. 98:8762–8767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwiatkowski, D.J., H. Zhang, J.L. Bandura, K.M. Heiberger, M. Glogauer, N. el-Hashemite, and H. Onda. 2002. A mouse model of TSC1 reveals sex-dependent lethality from liver hemangiomas, and up-regulation of p70S6 kinase activity in Tsc1 null cells. Hum. Mol. Genet. 11:525–534. [DOI] [PubMed] [Google Scholar]
- Lamb, R.F., C. Roy, T.J. Diefenbach, H.V. Vinters, M.W. Johnson, D.G. Jay, and A. Hall. 2000. The TSC1 tumour suppressor hamartin regulates cell adhesion through ERM proteins and the GTPase Rho. Nat. Cell Biol. 2:281–287. [DOI] [PubMed] [Google Scholar]
- Manning, B.D., A.R. Tee, M.N. Logsdon, J. Blenis, and L.C. Cantley. 2002. Identification of the tuberous sclerosis complex tumor suppressor gene product tuberin as a target of the phosphoinositide 3-kinase/Akt pathway. Mol. Cell. 10:151–162. [DOI] [PubMed] [Google Scholar]
- Onda, H., A. Lueck, P.W. Marks, H.B. Warren, and D.J. Kwiatkowski. 1999. TSC2(+/−) mice develop tumors in multiple sites that express gelsolin and are influenced by genetic background. J. Clin. Invest. 104:687–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter, C.J., H. Huang, and T. Xu. 2001. Drosophila tsc1 functions with tsc2 to antagonize insulin signaling in regulating cell growth, cell proliferation, and organ size. Cell. 105:357–368. [DOI] [PubMed] [Google Scholar]
- Potter, C.J., L.G. Pedraza, and T. Xu. 2002. Akt regulates growth by directly phosphorylating Tsc2. Nat. Cell Biol. 4:658–665. [DOI] [PubMed] [Google Scholar]
- Pullen, N., P.B. Dennis, M. Andjelkovic, A. Dufner, S. Kozma, B.A. Hemmings, and G. Thomas. 1998. Phosphorylation and activation of p70s6k by PDK1. Science. 279:707–710. [DOI] [PubMed] [Google Scholar]
- Radimerski, T., J. Montagne, F. Rintelen, H. Stocker, J. van Der Kaay, C.P. Downes, E. Hafen, and G. Thomas. 2002. dS6K-regulated cell growth is dPKB/dPI(3)K-independent, but requires dPDK1. Nat. Cell Biol. 4:251–255. [DOI] [PubMed] [Google Scholar]
- Reif, K., B.M. Burgering, and D.A. Cantrell. 1997. Phosphatidylinositol 3-kinase links the interleukin-2 receptor to protein kinase B and p70 S6 kinase. J. Biol. Chem. 272:14426–14433. [DOI] [PubMed] [Google Scholar]
- Tapon, N., N. Ito, B.J. Dickson, J.E. Treisman, and I.K. Hariharan. 2001. The Drosophila tuberous sclerosis complex gene homologs restrict cell growth and cell proliferation. Cell. 105:345–355. [DOI] [PubMed] [Google Scholar]
- van Slegtenhorst, M., M. Nellist, B. Nagelkerken, J. Cheadle, R. Snell, A. van den Ouweland, A. Reuser, J. Sampson, D. Halley, and P. van der Sluijs. 1998. Interaction between hamartin and tuberin, the TSC1 and TSC2 gene products. Hum. Mol. Genet. 7:1053–1057. [DOI] [PubMed] [Google Scholar]
- Vlahos, C.J., W.F. Matter, K.Y. Hui, and R.F. Brown. 1994. A specific inhibitor of phosphatidylinositol 3-kinase, 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one (LY294002). J. Biol. Chem. 269:5241–5248. [PubMed] [Google Scholar]
- von Manteuffel, S.R., P.B. Dennis, N. Pullen, A.C. Gingras, N. Sonenberg, and G. Thomas. 1997. The insulin-induced signalling pathway leading to S6 and initiation factor 4E binding protein 1 phosphorylation bifurcates at a rapamycin-sensitive point immediately upstream of p70s6k. Mol. Cell. Biol. 17:5426–5436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young, J., and S. Povey. 1998. The genetic basis of tuberous sclerosis. Mol. Med. Today. 4:313–319. [DOI] [PubMed] [Google Scholar]