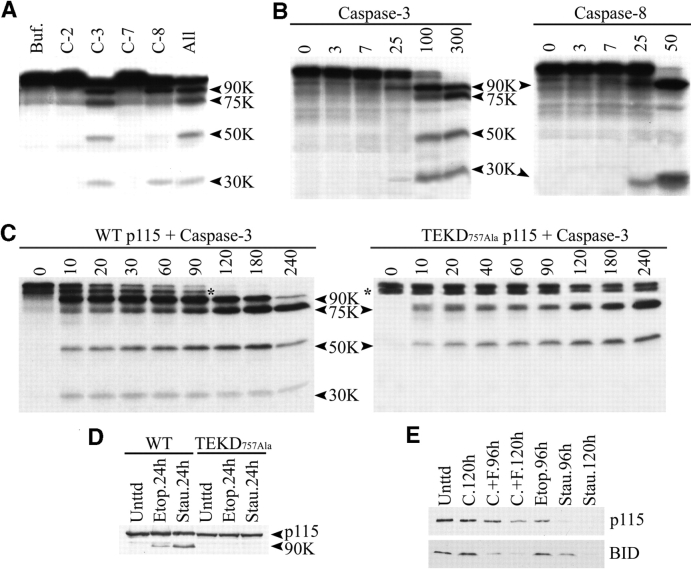
Figure 3.
p115 is cleaved by caspases-3 in vitro and in vivo at residue TEKD 757 to generate the 90- and 30-kD fragments. (A) In vitro–translated human p115 mRNA was incubated with various caspases for 1 h at 37°C. p115 was cleaved by purified caspase-3 into four fragments of 90, 75, 50, and 30 kD. Caspase-8 generated only the 90- and 30-kD fragments. (B) In vitro–translated bovine p115 mRNA was incubated with the same caspases as human p115. Numbers at the top indicate the concentration (nM) of caspase in each reaction. (C) In vitro–translated human p115 mRNA was incubated with caspase-3 for the indicated times (min). For wild-type p115 (left), the 90- and 30-kD fragments were rapidly generated after 10 min of incubation. In contrast, the 75- and 50-kD fragments required prolonged incubation before reaching a plateau. For the p115 TEKD757Ala mutant (right), the appearance of the 90- and 30-kD fragments was completely abrogated, whereas the 75- and 50-kD fragments were unaffected. Asterisks indicate an incomplete translation product of p115. (D) COS7 cells were transfected with NH2-terminal FLAG constructs of wild-type p115 or the p115 TEKD757Ala mutant. 24 h after transfection, apoptosis was induced with staurosporine or etoposide for the indicated times. Lysates were prepared and analyzed by Western blotting using polyclonal FLAG antibodies. Wild-type p115, unlike the p115 TEKD757Ala mutant, was cleaved to generate the 90-kD fragment. (E) MCF-7 cells were treated with staurosporine, etoposide, cycloheximide (C.), or agonistic anti-Fas antibodies plus cycloheximide (C.+F.) for the indicated times. Cell lysates were prepared and Western blot analysis was performed using antibodies to p115 and BID.