Figure 2.
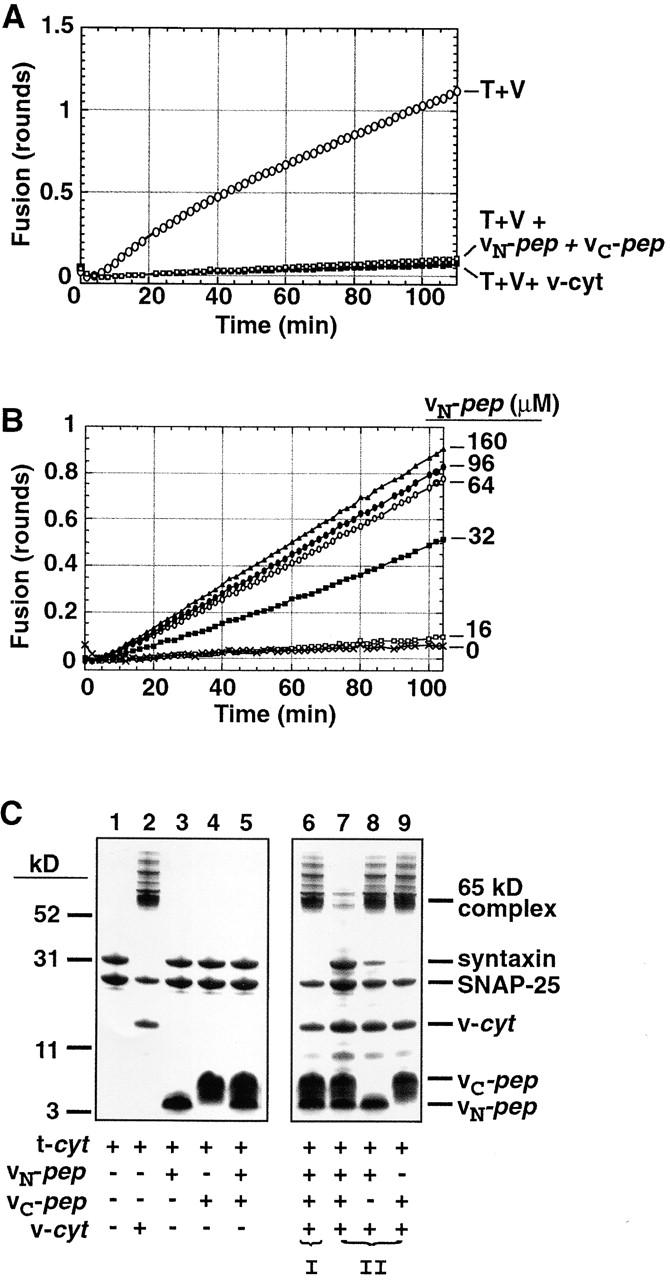
vN-pep and vC-pep bind the t-SNARE via the functionally relevant VAMP2 binding site. (A) vN-pep and vC-pep, when added together, compete with v-liposomes to inhibit fusion as effectively as v-cyt. Lipid-mixing fusion assays of t- and v-liposomes (T + V) were performed at 37°C as described in Materials and methods. Protein- and peptide-mediated inhibitions were tested by adding buffer (○), 50 μM vN-pep + 38 μM vC-pep (□), or 31 μM v-cyt (▪). (B) The VAMP2 coiled coil can be broken at the zero layer and still function in liposome fusion. vC-pep-cys was covalently modified with a C45 isoprenoid and incorporated into labeled liposomes. vC-pep-cys-C45 liposome fusion is dependent upon the concentration of vN-pep (no vN-pep, X; 16 μM, □; 32 μM, ▪; 64 μM, ○; 96 μM, •; 160 μM, ▵). (C) Together, the two peptides block SNARE complex formation. To test for the ability to form SDS-resistant complexes (indicated by the presence of the 65-kD band), 10 μg t-cyt was incubated with buffer (lane 1), 6 μg v-cyt (lane 2), 10 μg vN-pep (lane 3), 10 μg vC-pep (lane 4), or both peptides (lane 5) for 20 min at RT, and then immediately mixed with buffer without boiling and analyzed by SDS-PAGE and Coomassie blue staining. In order of addition experiments, to test for competition of binding at the VAMP2 binding site (lanes 6–9), t-cyt was preincubated with one component for 20 min before addition of the second component for 20 additional min. (I) t-cyt was incubated with v-cyt, and then both peptides. (II) t-cyt was incubated with one or both peptides, and then v-cyt. (Note: the SNAP-25 that does not become incorporated into the 65-kD SDS-resistant complex is derived from an excess of free SNAP-25 in the t-cyt preparation).
