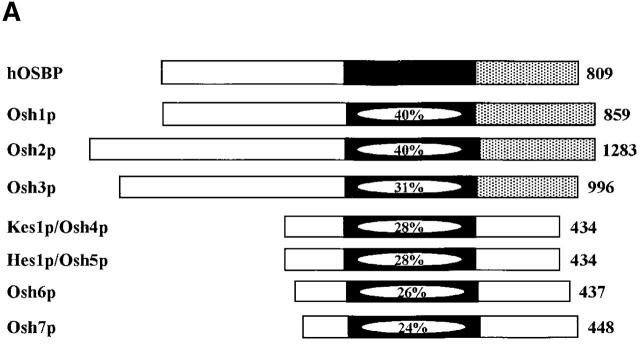
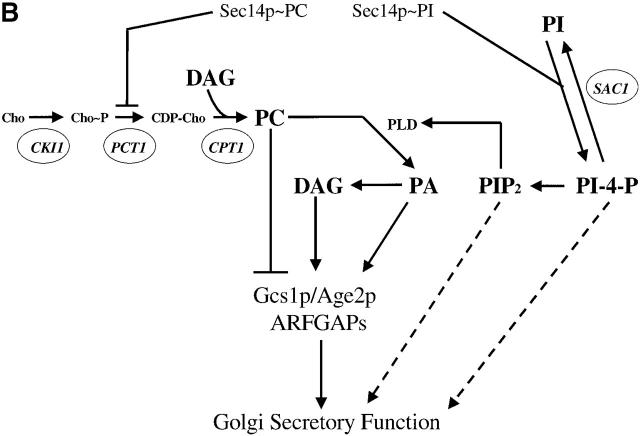
Figure 1.
Kes1p function and the Sec14p pathway. (A) The yeast OSBP family. The general organization of the seven yeast OSBP family members is given and compared with the human OSBP (hOSBP). Yeast proteins are identified at left by their common designations (see Beh et al., 2001). Regions homologous to the oxysterol binding domain of hOSBP are shown in black, and the primary sequence identities within that region are given. The three long yeast OSBPs (Osh1p, Osh2p and Osh3p) share homologies at their COOH-terminal domains with hOSBP (stippled areas), whereas these particular homologies are present in the four short yeast OSBPs (Kes1p, Hes1p, Osh6, and Osh7). Ultimate residues for each protein are numbered at right. (B) Pathway for Sec14p-dependent Golgi secretory function. Roles for phosphatidylcholine (PC), DAG, PA, PI(4)P, and PI(4,5)P2 in stimulating yeast Golgi complex secretory function have been proposed. PC, DAG, and PA are proposed to mediate combinatorial regulation of a pair of imperfectly redundant ARFGAPs (Gcs1p and Age2p) whose activity is required for Sec14p pathway function (Yanagisawa, L., and V.A. Bankaitus, unpublished data). The known execution points for relevant genes whose inactivation effects “bypass Sec14p” (i.e., CKI1, PCT1, CPT1, and SAC1) are shown. The execution point of the KES1 gene product in the Sec14p pathway is unknown.