Figure 4.
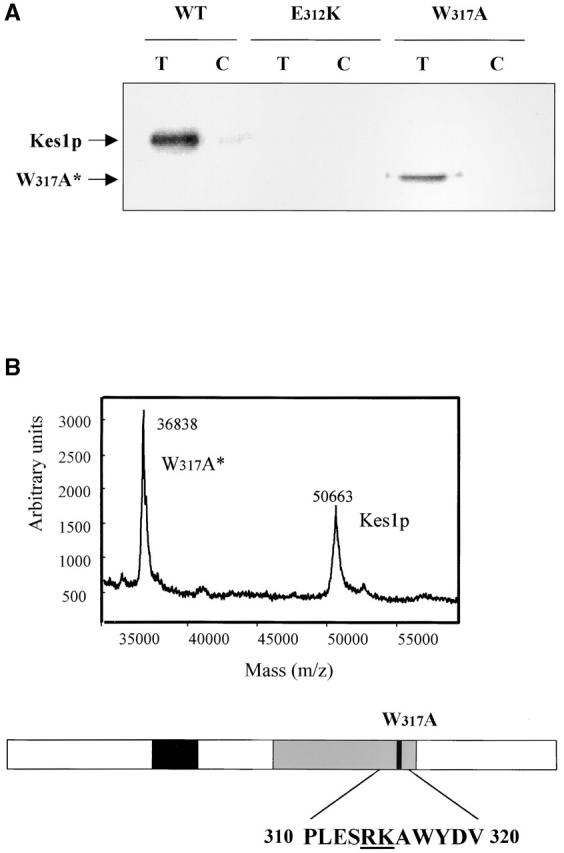
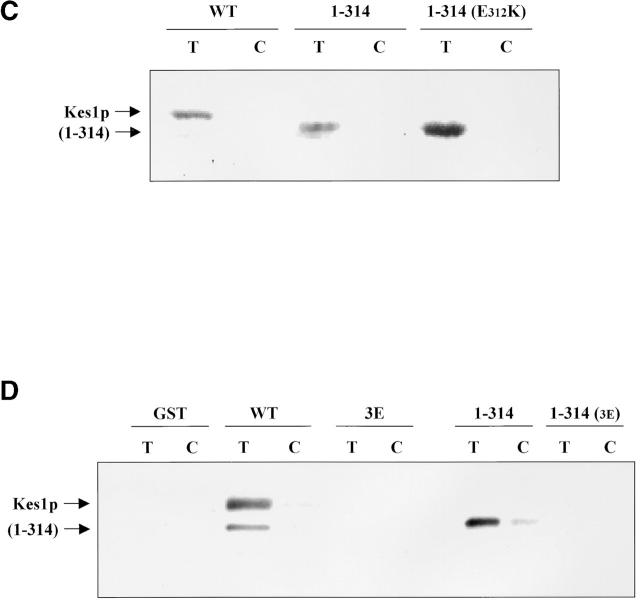
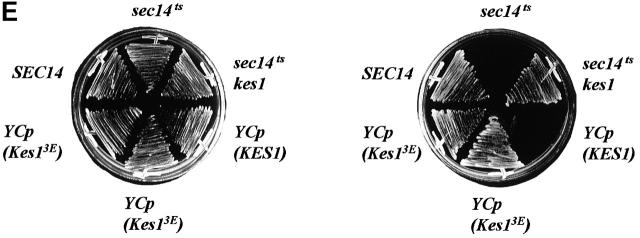
Kes1p PH domain mutations and PIP binding. (A) Mutations in Kes1p PH domain compromise PIP binding. Photoaffinity-labeling assays were performed with mutant Kes1ps using [3H]BZDC-PI(4,5)P2 as photo-probe. Covalent Kes1p–photo-probe adducts were resolved by SDS-PAGE and visualized by autoradiography. Kes1pE312K and full-length Kes1pW317A fail to bind [3H]BZDC-PI(4,5)P2. A proteolytic breakdown product of Kes1pW317A (Kes1pW317A*) regains PIP binding competence. (B) MALDI-TOF mass spectrometry of Kes1pW317A and Kes1pW317A*. The mass to charge ratios of full-length Kes1pW317A and the Kes1pW317A* truncation product are shown and the corresponding mass values for each are indicated. Below is an illustration of the Kes1p domain structure where the OSBP and PH domains are designated by the black and gray boxes, respectively. The location of the W317A missense substitution within the PH domain is shown by a black bar, and the surrounding wild-type sequence is displayed at bottom (W corresponds to W317). The R314K315 motif that defines the site of proteolysis that generates Kes1pW317A* is underlined. (C) COOH-terminal Kes1p truncation and PIP binding. Wild-type and mutant forms of Kes1p were genetically truncated after residue K314 to generate protein fragments consisting of otherwise wild-type primary sequence (Kes1p1–314). GST-tagged versions of Kes1p1–314 and Kes1pE312K* were purified and photolabeled with [3H]BZDC-IP3 in a mixed micelle system in the absence (T) or presence (C) of competitor PI(4,5)P2. Both full-length protein and fragments associate with [3H]BZDC-IP3 in a manner that is subject to displacement by PI(4,5)P2. (D) COOH-terminal truncation of Kes1p3E fails to rescue PIP binding. GST-tagged Kes1p and Kes1p3E, and the corresponding COOH-terminal truncation fragments Kes1p1–314 and Kes1p3E* were purified were photolabeled with [3H]BZDC-IP3 in a mixed micelle system without (T) and with (C) unlabeled PI(4,5)P2 competitor. Full-length Kes1p3E fails to bind photo-probe, and this failure is not rescued by Kes1p truncation. (E) PIP binding is essential for Kes1p function in vivo. Centromeric plasmids (YCp) that drive physiological levels of KES1 or kes1 3E expression were transformed into the kes1 strain CTY159. Transformants were streaked for isolation onto selective minimal media and incubated for 3 d at 26°C or 37°C. Complementation of kes1 defects is scored by failure of transformants to grow at 37°C.