Figure 1.
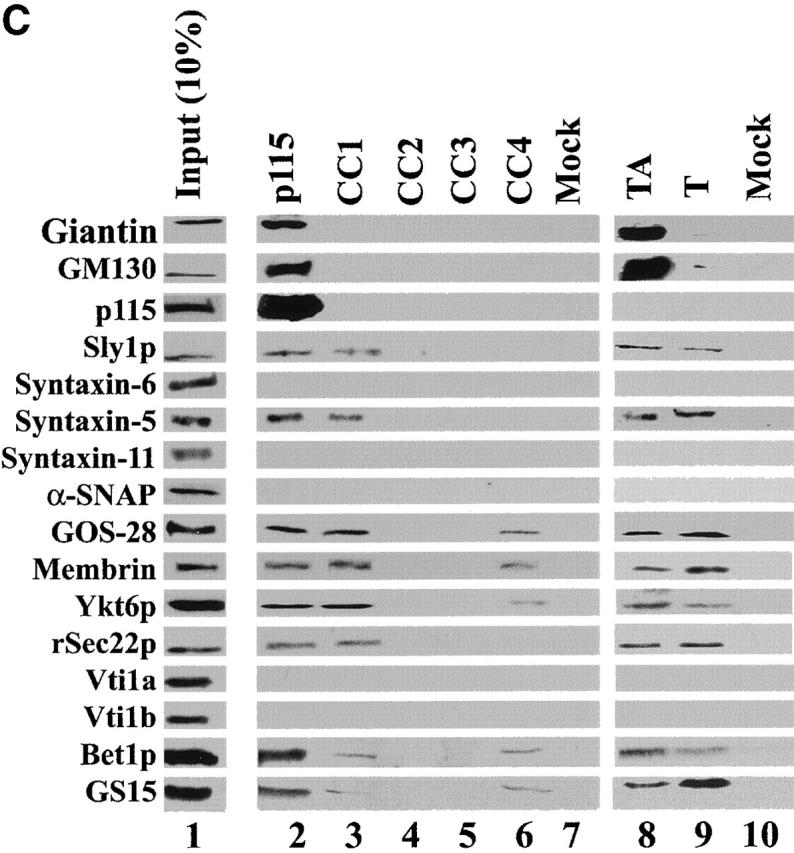
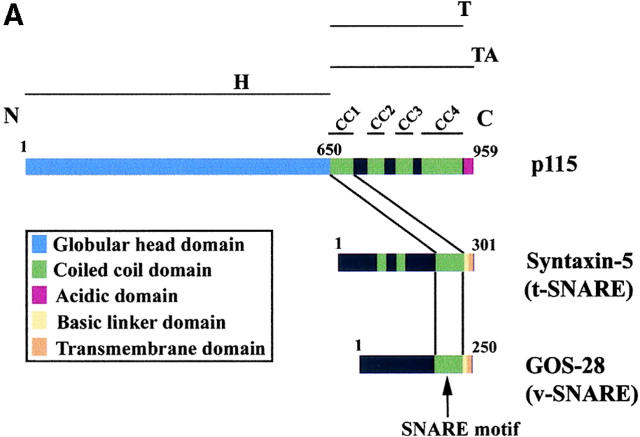
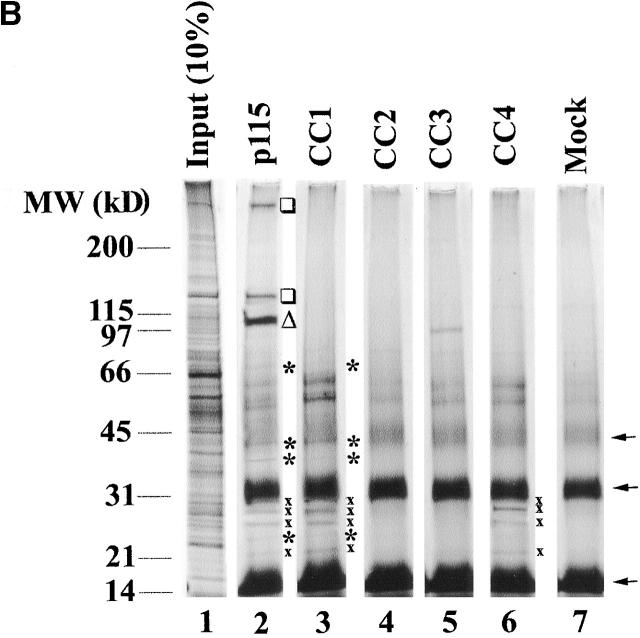
A SNARE motif-related region in p115 binds specific SNAREs. (A) The domain architecture of p115, syntaxin-5 (a t-SNARE), and GOS-28 (a v-SNARE). p115 consists of a globular head domain (H, blue), a tail domain (T) containing four coiled-coil domains (CC1-4, green), and an acidic COOH-terminal domain (A, red). SNAREs contain an ∼60 amino acid membrane proximal coiled-coil domain (green) termed the SNARE motif followed by a basic linker region (yellow) and a transmembrane domain (orange). t-SNAREs possess additional NH2-terminal coiled-coil regions. The first coiled-coil domain of p115 (CC1) displays weak homology to the SNARE motif (Weimbs et al., 1997). (B) RLGs (20 μg) were extracted with Triton X-100 buffer, clarified, and incubated with Neutravidin beads (mock) or beads bound to biotinylated p115 or CC1-4 peptides. Washed beads were eluted, and eluates were fractionated by SDS-PAGE and silver stained. Asterisks denote proteins selectively retained on p115 and CC1 beads but not others. Crosses denote proteins selectively retained on p115, CC1, and CC4 beads but not others. The triangle denotes p115 eluted from p115 beads. Squares denote proteins that correspond in size to Giantin (□, top) and GM130 (□, bottom), which are retained only on p115 beads. Arrows indicate Neutravidin breakdown products. (C) Immunoblot analyses of B. In addition, His-TA and His-T (0.5 μM) were incubated with clarified Golgi detergent extract, retrieved with Ni-NTA agarose, and processed as in B before immunoblot analysis.