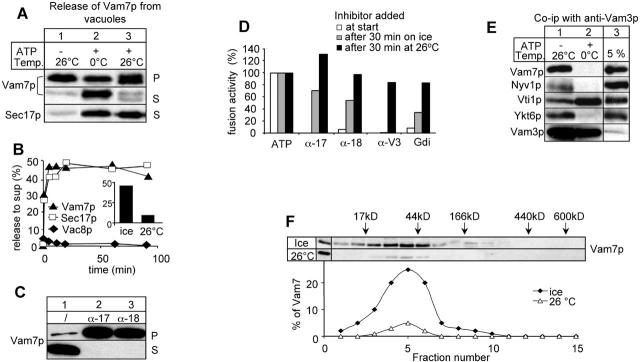
Figure 1.
Priming-dependent release of Vam7p on ice. (A) Vam7p is released from the vacuoles in an ATP-dependent reaction. BJ3505 vacuoles (12 μg) were incubated in a 60-μl reaction for 60 min on ice or at 26°C in reaction buffer containing His6-Sec18p, CoA (10 μM), and ATP as indicated. Then vacuoles were separated from the supernatant (5 min, 20,000 g, 4°C), and the reaction supernatant was precipitated by addition of TCA (13% [vol/vol]) (Ungermann et al., 1998a). Vacuole pellets and precipitated proteins from the supernatant were solubilized in sample buffer and analyzed by SDS-PAGE and immunoblotting. Immunoblots were decorated with indicated antibodies. (B) Release of Vam7p and Sec17p on ice is immediate. BJ3505 vacuoles (12 μg) were incubated on ice, separated into vacuole and supernatant fraction, and processed as in A. Immunoblots of the supernatant fractions were quantified and plotted versus time. The inset shows a quantification of Vam7p in the reaction supernatant after a 60-min incubation on ice or at 26°C. (C) Priming inhibitors block Vam7p release. BJ3505 VAM7-HA vacuoles (12 μg) were incubated in the presence of ATP and IgGs to Sec17p and Sec18p where indicated. Pellet and supernatant fractions were analyzed with HA antibodies to detect Vam7p. HA-tagged Vam7p was used to avoid interference with the added IgGs during immunoblot decoration. (D) Incubation with ATP on ice allows bypass of priming. BJ3505 and DKY6281 vacuoles (6 μg) were incubated in the presence of ATP at the indicated temperature. Inhibitors (α-Sec17p, α-Sec18p [200 μg/ml], α-Vam3p [50 μg/ml], and Gdi1p [300 μg/ml]) were added to the reactions either right away (white bars), after a 30-min ice incubation (gray bars), or after a 30-min incubation at 26°C. Each reaction was then incubated for an additional 60 min at 26°C and then assayed for alkaline phosphatase activity. (E) Vam7p is released from the cis-SNARE complex. BJ3505 vacuoles (36 μg) were incubated in a 180-μl reaction on ice for 10 min. Vacuoles were reisolated (5 min, 20,000 g, 4°C) and solubilized in lysis buffer (150 mM KCl, 0.5% Triton X-100, 20 mM Hepes/KOH, pH 7.4, 1xPIC, and 1 mM PMSF) for 10 min at 4°C and centrifuged (20,000 g, 10 min, 4°C). The soluble detergent extract was added to protein A–Sepharose–coupled antibodies to Vam3p (Ungermann et al., 1998a) and incubated for 2 h at 4°C on a nutator. Reisolated beads were washed once with lysis buffer and once with lysis buffer containing 500 mM KCl. Bound proteins were eluted from the beads with 1 ml of 0.1 M glycine, pH 2.6. The eluate was TCA precipitated and analyzed by SDS-PAGE, Western transfer and immunoblotting with antibodies against the indicated proteins. (F) Released Vam7p is a monomer. BJ 3505 vacuoles (60 μg) were incubated in the presence of ATP on ice or at 26°C as described in the legend to Fig. 1 A. The supernatant was loaded onto a continuous glycerol gradient (10–30% in PS buffer supplemented with 150 mM KCl). The samples were centrifuged at 40,000 rpm in a SW40 rotor for 18 h at 4°C. Fifteen 500-μl fractions were collected, TCA precipitated, and analyzed by SDS-PAGE and immunoblot as before.