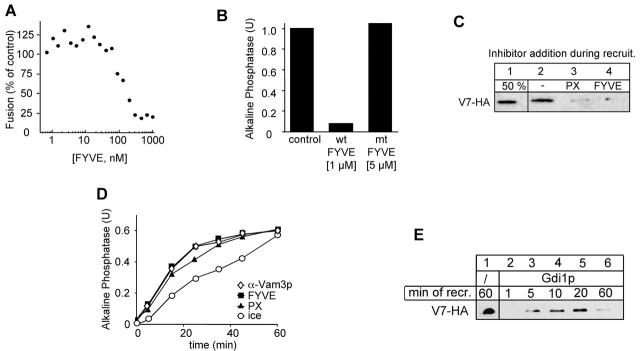
Figure 6.
Masking of PtdIns 3-P reveals a specific function of the PX domain. (A) Inhibition of fusion by the recombinant FYVE domain. GST-2xFYVE (as described in Materials and methods) was added at the indicated concentration to the fusion reaction. Fusion was assayed after 90 min at 26°C. (B) Inhibition by the FYVE domain is specific. The indicated concentration of wild-type FYVE domain (1 μM) or an inactive mutant (C215S; 5 μM) was added to a fusion reaction. Alkaline phosphatase activity was determined after 90 min at 26°C. (C) Both PX and FYVE domain block the recruitment of Vam7p. Acceptor vacuoles were preincubated on ice with ATP for 30 min, then reisolated (5 min, 20,000 g, 4°C), and incubated with recombinant FYVE (1 μM) or PX (15 μM) protein. The recruitment of Vam7-HA and the analysis was done as described in the legend to Fig. 3. (D) Kinetic analysis of the PX and FYVE inhibition. The experiment was performed as described in the legend to Fig. 4 B. At the indicated time points, FYVE protein (1 μM) or the PX domain (15 μM) were added. (E) Binding and release of Vam7p from Gdi1p-treated vacuoles. Wild-type acceptor vacuoles were primed on ice, then pretreated with Gdi1p (see Fig. 5 C), resuspended with Vam7-HA containing supernatant, and incubated at 26°C. At the indicated times, aliquots were removed and separated into pellet and supernatant fraction. Analysis of the pellet was done as described above.