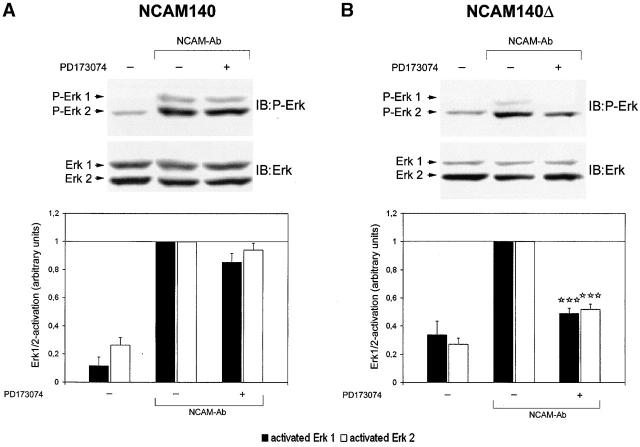
Figure 6.
Deletion of NCAM140 palmitoylation sites results in a shift of NCAM140-mediated signal transduction from FGF receptor–independent toward FGF receptor–dependent signaling. CHO cells were transfected with NCAM140 or NCAM140Δ and assayed for ERK1/2 activation by immunoblot analysis. (A) Immunoblot of NCAM140-transfected CHO cell lysates analyzed for phosphorylated ERK1/2 (top). Bar graph shows ERK1/2 phosphorylation by densitometric analysis of immunoblots (bottom). Stimulation of NCAM140-transfected CHO cells results in phosphorylation of ERK1/2, which is not attenuated by the FGF receptor inhibitor PD173074, indicating an FGF receptor–independent signaling of NCAM140. (B) Immunoblot of NCAM140Δ-transfected CHO cell lysates analyzed for phosphorylated ERK1/2 (top). Bar graph shows ERK1/2 phosphorylation revealed by densitometric analysis of immunoblots (bottom). Stimulation of NCAM140Δ also results in phosphorylation of ERK1/2, which in contrast to NCAM140 is impaired by PD173074. The bars show mean and SEM of 8–12 independent experiments. Asterisks denote statistical significance (paired t test) of attenuation of ERK1/2 phosphorylation due to treatment with the FGF receptor inhibitor compared with NCAM-mediated ERK1/2 activation in transfected CHO cells, which were not treated with PD173074 (***p < 0.0005). IB, immunoblot for the molecules indicated.