Figure 4.
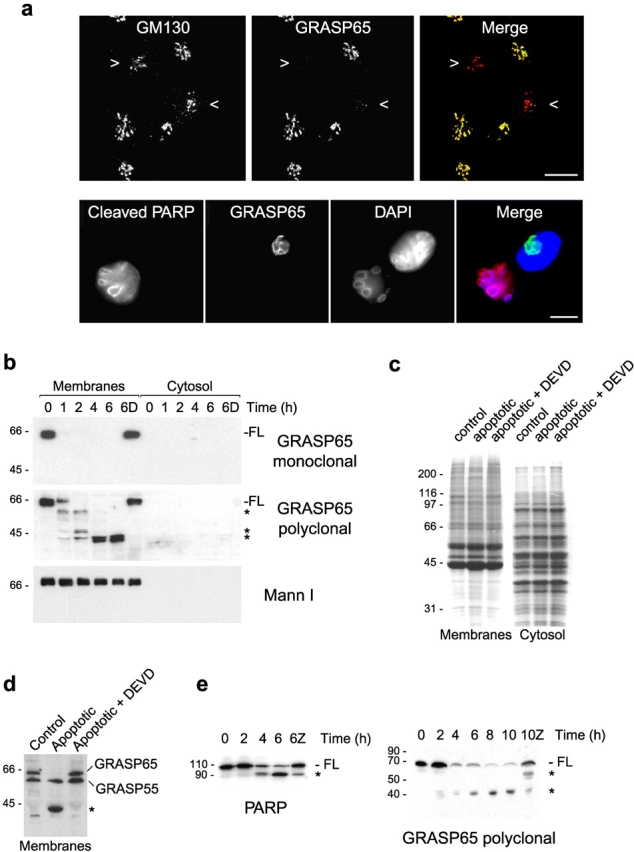
GRASP65 is a substrate for caspase-mediated proteolysis during apoptosis. (a) NRK cells treated with 1 μM staurosporine for 4 h were processed for immunofluorescence microscopy. (Top) Cells were double labeled with polyclonal antibodies to GM130 and a monoclonal antibody to GRASP65. Regions of overlap between GM130 (red) and GRASP65 (green) are shown in the merge in yellow. Apoptotic cells were identified using DAPI (unpublished data) and are indicated by arrowheads. (Bottom) Cells were labeled with polyclonal antibodies to cleaved PARP, a monoclonal antibody to GRASP65, and DAPI as indicated. A merged image showing cleaved PARP (red), GRASP65 (green), and DAPI (blue) is on the right. (b) Purified Golgi membranes were incubated at 37°C with apoptotic HeLa cytosol in the absence or presence of 2 μM Ac-DEVD-CHO (D) for the times indicated. Membranes were pelleted, and membrane and supernatant (cytosol) fractions were analyzed by immunoblotting with monoclonal or polyclonal (FBA31) antibodies to GRASP65 or antibodies to mannosidase I. (c and d) Golgi membranes were incubated with HeLa control cytosol or apoptotic cytosol in the absence or presence of 2 μM Ac-DEVD-CHO for 4 h at 37°C. Membrane and cytosol fractions were analyzed by Coomassie blue staining (c) or immunoblotting with an antipeptide antibody that recognizes both GRASP55 and GRASP65 (d). (e) NRK cells treated with 1 μM staurosporine in the absence or presence of 50 μM zVAD.fmk (Z) for the times indicated were analyzed by immunoblotting with monoclonal and polyclonal (CT) antibodies to PARP and GRASP65, respectively. (b, d, and e) Full-length (FL) and caspase cleavage products (asterisks) are marked. Bars, 10 μm.
