Figure 8.
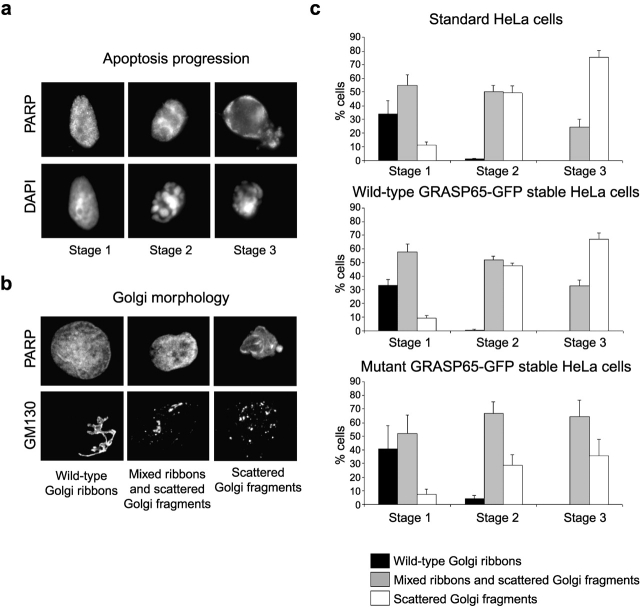
GRASP65 cleavage is required for fragmentation of the Golgi ribbon during apoptosis. The progress of Golgi fragmentation was analyzed in standard HeLa cells and cells stably expressing wild-type GRASP65-GFP or caspase-resistant mutant GRASP65-GFP. Staurosporine-treated cells (6 h) were fixed and then stained with a monoclonal antibody against GM130 (detected with Alexa 488–conjugated secondary antibody) and caspase-cleaved PARP (detected with Alexa 594 secondary antibody). (a) Cells were categorized for apoptotic progression (stage 1, PARP-positive nucleus with normal uncondensed chromatin; stage 2, PARP-positive nucleus with condensed chromatin; stage 3, highly condensed chromatin and PARP staining throughout the cell). (b) The condition of the Golgi apparatus was then assessed in apoptotic cells according to the parameters shown. (c) The proportion of cells displaying each category of Golgi morphology was quantitated in each of the three stages of apoptosis. All counting was performed blind. Means ± SEM are shown for three independent experiments. The total number of cells counted for each cell type was between 108–144 (stage 1), 358–392 (stage 2), and 35–72 (stage 3). Using the chi-square likelihood ratio contingency test, there was no significant difference at any stage between cells stably expressing wild-type GRASP65-GFP and the parental cells. For all experiments, there was a significant difference in the percentage of cells with stage 3 fragmented Golgi between cells expressing mutant GRASP65-GFP and either the parental cells (P = 0.001) or the wild-type GRASP65-GFP–expressing cells (P < 0.02). For stage 2, there was a significant difference in two out of three experiments between the mutant GRASP65-GFP cells and the parental cells and in all experiments between the mutant and wild-type GRASP65-GFP cells (P < 0.0167), whereas for stage 1 this was true in one out of three experiments.