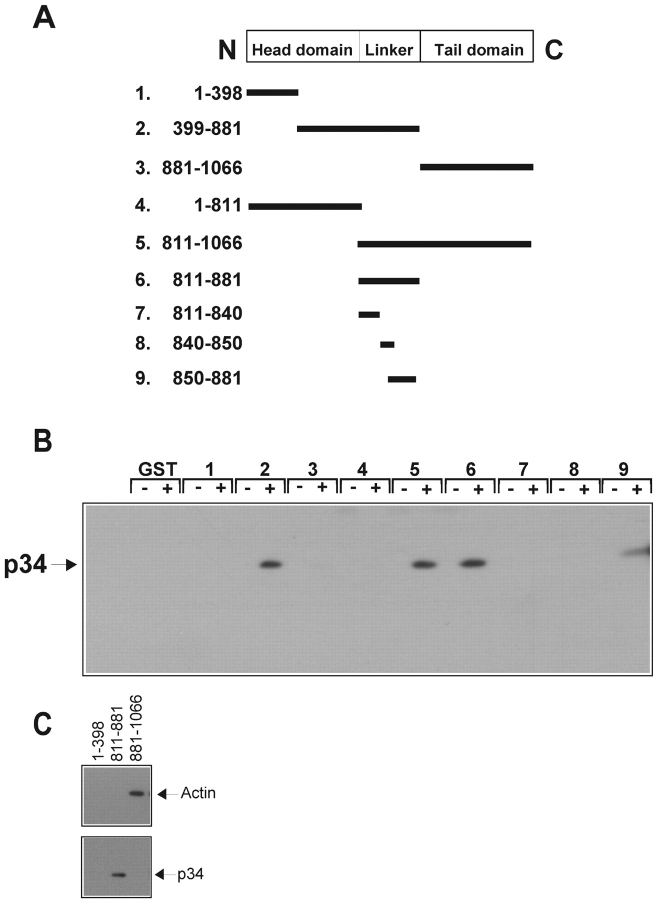
Figure 6.
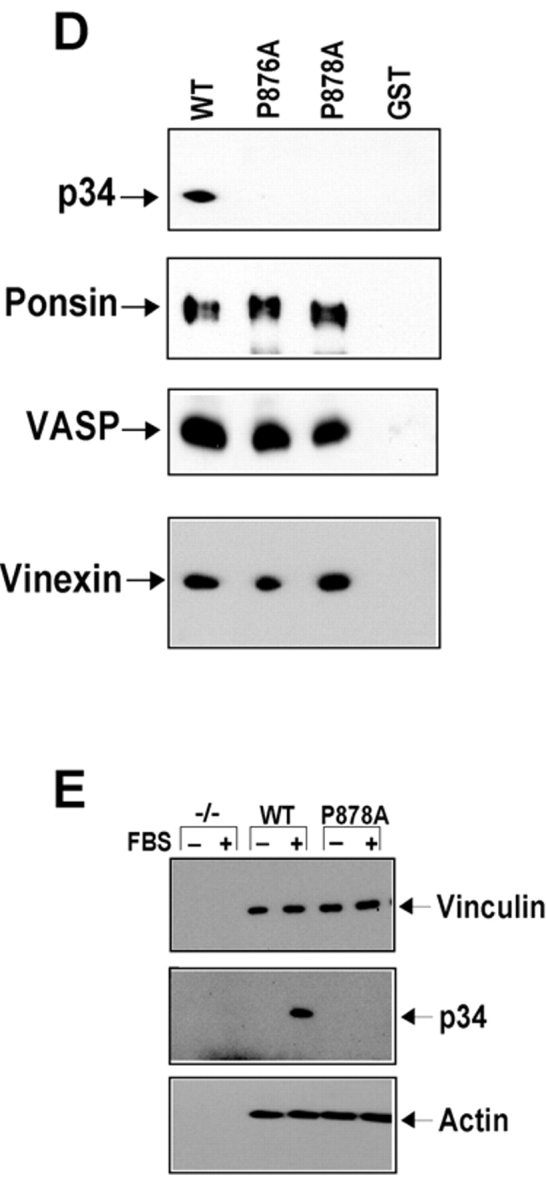
Mapping the Arp2/3 complex binding site of vinculin. (A) Linear schematic is shown of vinculin and the fragments of vinculin as fusion proteins of GST (GST–VIN). (B and C) Association of the Arp2/3 complex with GST–VIN proteins. Thirty micrograms of fusion protein attached to beads were incubated for 1.5 h in the absence (−) or presence (+) of 10 μl of a crude platelet fraction. The beads were sedimented, washed, and subjected to Western blot analysis as described. Note that in C, the binding of the Arp2/3 complex to vinculin fragments does not involve binding to actin. (D) Substitution of proline 878 or 876 with alanine prevents binding of the Arp2/3 complex to vinculin in vitro. 30 μg of GST or GST fusions of VIN 811–881, VIN 811–881 with a proline to alanine mutation at 876 (P876A) or at 878 (P878A) were examined for their ability to retrieve the Arp2/3 complex, ponsin, vinexin, or VASP from cell lysates obtained from platelets, MDCK, C2C12, or A431 cells, respectively. Different cell types were used for preparing the lysates because of failure to detect the antigens in some cell types. (E) Full-length vinculin harboring a proline to alanine mutation at 878 prevents recruitment of the Arp2/3 complex, but has no effect on actin binding. Serum-starved, Vin−/− MEFs expressing an empty vector (−/−), full-length vinculin (WT), or full-length vinculin with a P878A substitution (P878A) were left resting (−) or stimulated (+) with 10% FBS for 5 min, vinculin immunoprecipitates were obtained and analyzed.