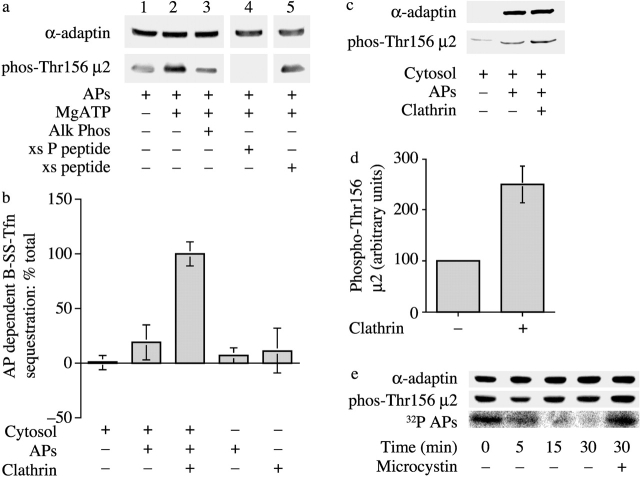
Figure 2.
Clathrin activation of the μ2 kinase is required for coated pit function in vitro. (a) Characterization of phospho-Thr156 μ2 antibodies. APs were incubated with Mg2+ATP for 15 min at 30°C, and then in the presence or absence of calf alkaline phosphatase for 10 min at 30°C before immunoblotting. The blots were probed with antibodies that recognize the brain-specific insert of α-adaptin or with antibodies generated against phospho-Thr156 μ2. Lane 4, anti-phospho-Thr156 μ2 antibodies were incubated in the presence of a 100-fold molar excess of phosphorylated peptide used to generate the antibodies. Lane 5, anti-phospho-Thr156 μ2 antibodies were incubated with 100-fold molar excess of the nonphosphorylated peptide. (b) The sequestration of biotinylated transferrin into new clathrin coated pits was measured by inaccessibility to avidin with the modifications described in Materials and methods. Results are expressed as the mean ± SEM of three experiments. (c) Permeabilized cell membranes incubated as indicated were reisolated and subjected to immunoblotting with antibodies against the brain-specific insert of α-adaptin or with anti-phospho-Thr156 μ2 antibodies. (d) A histogram showing quantitation of the levels of μ2 phosphorylation in permeabilized cell membranes in the presence or absence of clathrin. Results are expressed as the mean ± SD of three separate experiments. (e) μ2 undergoes cycles of phosphorylation during cargo sequestration. Permeabilized cell assay mixes were incubated APs that were phosphorylated before either with ATP (middle) or [32P]ATP (bottom) and in the absence or presence of microcystin as indicated. The membranes were reisolated at the indicated times, and after SDS-PAGE, the amount of phosphorylated μ2 was measured using phospho-Thr156 μ2 antibodies or by autoradiography.