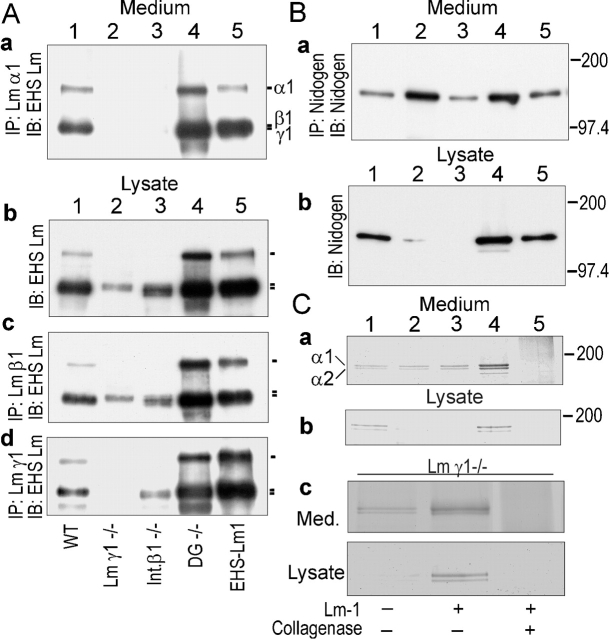
Figure 9.
Expression and accumulation of basement membrane components. Conditioned media (10 ml from the last 2 d) and EBs were collected from cultures of wild-type, β1-integrin–null, γ1-laminin–null, and dystroglycan-null ES cells maintained for 7 d. The cell pellets were extracted with 0.5 ml of lysis buffer, 0.5 ml conditioned medium, or 0.15 ml EB lysates were incubated with antibody specific for the laminin-α1 (anti-RG50), β1 (anti-E4), or γ1 (rat anti–mouse γ1 chain mAb), and then pulled down with protein A or protein G coupled to agarose beads (immunoprecipitation [IP]). Alternatively, the extract or medium fraction was analyzed directly with EHS laminin-1-specific pAb in immunoblots (IB). (A) Laminin. Medium (IP/IB) and embryoid body cell pellet (IB or IP/IB). Samples correspond to EBs prepared from wild-type (lane 1), γ1-laminin–null (lane 2), β1-integrin–null (lane 3), and dystroglycan-null (lane 4) ES cells, shown in comparison to purified EHS laminin-1 (lane 5). (B) Nidogen. Media and extracted EB pellets were analyzed in immunoprecipitates/immunoblots with specific antibody for nidogen as follows: wild-type (lane 1), γ1-laminin–null (lane 2), β1-integrin–null (lane 3), dystroglycan-null (lane 4), and nidogen standard (lane 5). (C) Type IV collagen-specific antibody was used to immunoprecipitate the collagen from media and EB fractions followed by reducing SDS-PAGE and Coomassie blue staining. Type IV collagen immunoprecipitated from wild-type conditioned medium or EBs could be digested with bacterial collagenase (lane 5).