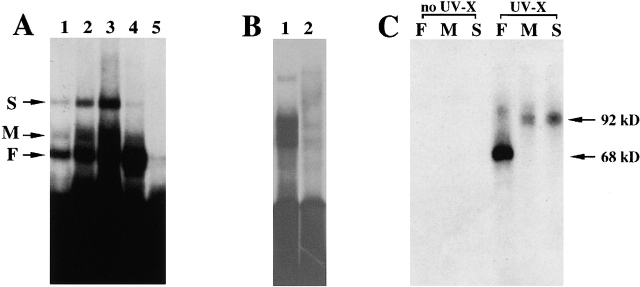
Figure 1.
Characterization of proteins binding to the zipcode in chicken embryo brain and fibroblast extracts. (A). Complexes formed from brain and fibroblasts. [32P]-labeled transcripts encoding the 54 nts zipcode of the 3′ UTR of chicken β-actin mRNA were incubated with aliquots of chicken embryo brain and fibroblast protein extracts, followed by incubation with RNase T1 (50 U/ml) and heparin (5 mg/ml). RNA–protein complexes were resolved in 4% polyacrylamide native gels that were then dried and exposed to Kodak X-Ray film. (Lanes 1, 2, and 3) [32P]-labeled transcript incubated with 1-, 2-, and 5-μl brain extracts, respectively. (Lane 4) [32P]-labeled transcript incubated with 5 μl fibroblast extract. (Lane 5) [32P]-labeled transcript incubated with 5-μl brain extract in the presence of 100× excess molar amounts of unlabeled 3′ UTR of actin mRNA transcripts. The arrows point to the slow (S), medium (M), and fast-migrating (F) RNA–protein complexes. (B) Specificity of the complexes for the zipcode. (Lane 1) Zipcode incubated with brain extract. (Lane 2) Mutant zipcode incubated with brain extract. (C) UV crosslinking assay. [32P]-labeled transcripts were incubated with brain extract, treated with RNase T1, and exposed to UV light for 3 min at a distance of 1 cm. Each of the RNA–protein complexes (F, M, and S) was identified and excised from a 4% polyacrylamide native gel, soaked in SDS buffer, and resolved in 10% SDS-PAGE. (Left three lanes) Excised RNA–protein complexes F, M, and S that were not UV crosslinked. (Right three lanes) Excised RNA–protein complexes F, M, and S that were crosslinked. The arrows indicate the protein mobilities with labeled RNA bound.