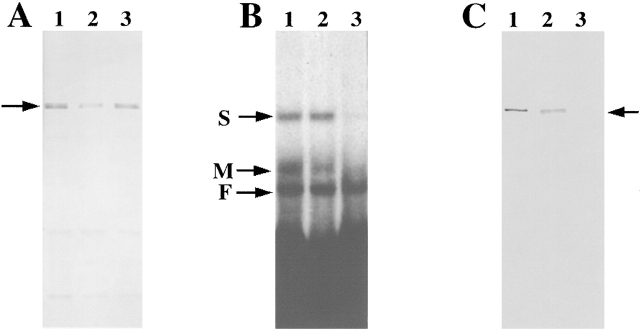
Figure 4.
Characterization of antibodies to ZBP2. Antibodies against ZBP2 were made by injecting SDS-PAGE gel purified ZBP2 into rats. (A) Western blotting to ZBP2. (Lane 1) Crude brain extract. (Lane 2) Crude fibroblast extract. (Lane 3) 1.0 M KCl affinity- purified protein fraction of brain extract. The arrow indicates ZBP2. (B) Antiserum against ZBP2 or preimmune serum was incubated with brain extract for 2 h followed by incubation with agarose-protein G beads for 2 h at 4°C. The supernatants, after centrifugation at 1,500 rpm for 10 min, were analyzed by motility shift assay for their ability to form an RNA–protein complex. (Lane 1) Control brain extract. (Lane 2) Brain extract incubated with rat preimmune serum. (Lane 3) Brain extract incubated with anti-ZBP2 serum. Arrows denote the RNA–protein complexes identified in Fig. 1 A. (C) The same samples as in B were used for Western blotting. (Lane 1) Control brain extract. (Lane 2) Brain extract incubated with rat preimmune serum. (Lane 3) brain extract immunodepleted using anti-ZBP2 serum. Arrow indicates ZBP2.