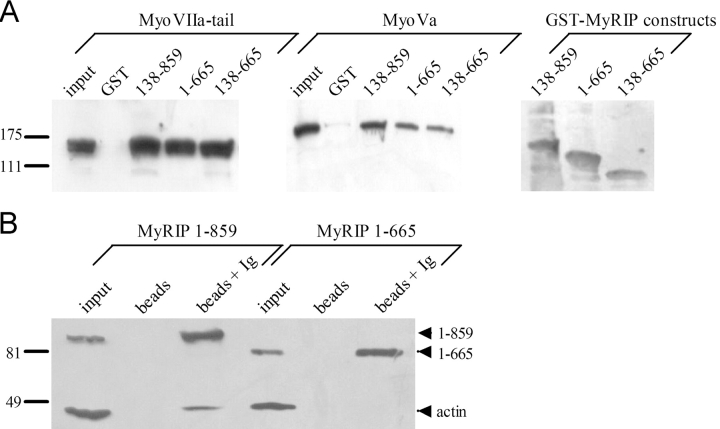
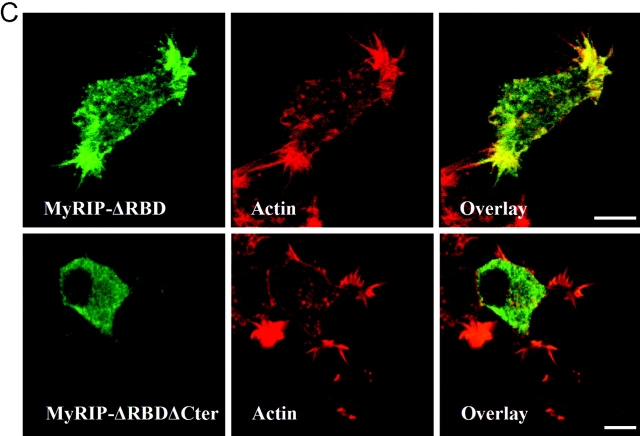
Figure 4.
Interaction of MyRIP with myosin-Va and actin. (A) Binding of myosin-VIIa tail (left) or myosin-Va (middle) to purified GST or GST–MyRIP constructs immobilized on glutathione–Sepharose beads. Transfected COS-7 cells and nontransfected PC12 cells were used as a source of myosin-VIIa and myosin-Va, respectively. Cellular levels of myosin-VIIa and myosin-Va are shown in the first lane of each panel (input, 10% of the volume used in the experiment). The position of molecular weight markers is shown on the left side (×10−3). The same blots were stripped and reprobed with an anti-GST monoclonal antibody to reveal the MyRIP constructs bound to the beads (right). Note that deletion of the COOH-terminal region of MyRIP had little effect on the interaction with myosins. Similar results were obtained in another experiment. (B) The COOH-terminal region of MyRIP is required for actin binding. COS-7 cells were transfected with vectors encoding full-length MyRIP (1–859) or a deletion mutant (1–665). The proteins expressed were immunoprecipitated with anti-MyRIP antibody–conjugated protein A–Sepharose (beads + Ig). Coimmunoprecipitated actin was revealed with a monoclonal antibody. Beads without antibody were used as control (beads). The amount of expressed MyRIP proteins used in the immunoprecipitation is shown on the left (input, 1/8 of the volume used). Similar results were obtained in another experiment. (C) Importance of the COOH-terminal region of MyRIP for its colocalization with F-actin. PC12 cells were transfected with MyRIP-ΔRBD (138–859) (top) or MyRIP-ΔRBD-ΔCter (138–665) (bottom). 3 d later, cells were stained with anti–myc tag antibodies (left) or rhodamine-phalloidin (middle) and imaged by confocal immunofluorescence. The overlaid images (right) show that MyRIP-ΔRBD, but not MyRIP-ΔRBD-ΔCter, colocalizes with F-actin.