Figure 5.
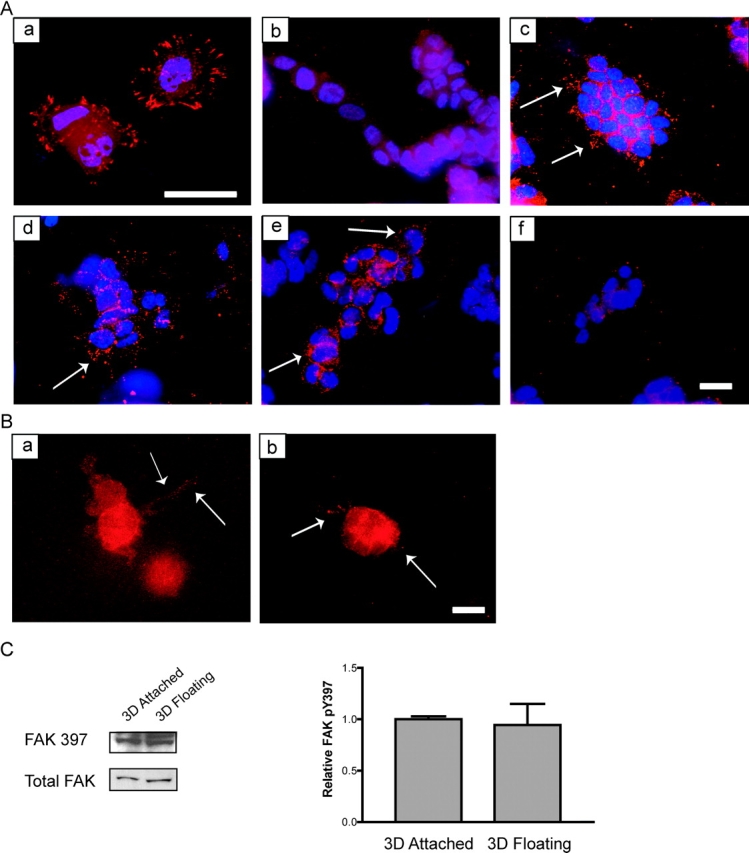
The rigidity of the matrix and cellular contractility regulates the localization of FAK phosphorylated at Y397 at 3D matrix adhesions. (A) FAK phosphorylated at Y397 is localized to focal adhesions when T47D cells are plated on 2D collagen-coated coverslips (a). In a floating 3D gel, FAK phosphorylation at Y397 is minimal (b). Cells cultured in an attached 3D collagen gel localized FAK pY397 to punctate adhesions (c). Cells in floating 3D gels treated with C3 (10 μg/ml) (d) or Y27632 (10 μM) (e) localized FAK pY397 to small 3D matrix adhesions (see arrows), whereas cells treated with ML7 (10 μM) lost this localization (f). FAK pY397 is shown in red, and nuclei are shown in blue. Bar: (a) 25 μm; (b–f) 50 μm. (B) Cells treated with BDM (20 mM) (a) or H7 (300 μM) (b) also localized FAK pY397 to punctuate matrix adhesions. Bar, 50 μm. (C) The localization, and not phosphorylation, of FAK Y397 is regulated by ECM rigidity. Western blot analysis of FAK pY397 in attached vs. floating collagen gels (1.3 mg/ml) show statistically similar (P = 0.8014) levels of phosphorylation and FAK expression. The quantitation represents four individual experiments (right).
