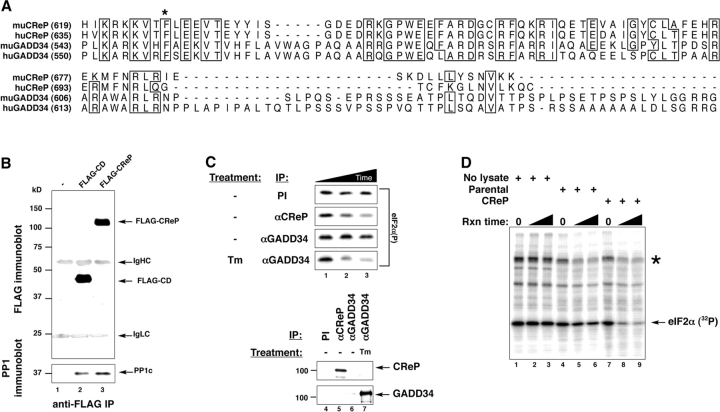
Figure 3.
CReP is a regulatory subunit of an eIF2α holophosphatase complex with similarity to GADD34 in its COOH-terminal, PP1c–binding region. (A) Alignment of the predicted amino acid sequence of the COOH termini of mouse (mu) and human (hu) CReP and GADD34. The asterisk indicates the phenylalanine residue conserved in all PP1 regulatory subunits that is required for interaction with PP1c. (B) Immunoblot of FLAG-epitope tagged CReP COOH-terminal fragment encoded by the CD retrovirus and full-length CReP immunoprecipitated from lysates of transfected 293T cells (top). Immunoblot of endogenous PP1c in the immunoprecipitates (bottom). (C) Autoradiogram of an in vitro dephosphorylation assay of 32P-radiolabeled eIF2α on serine 51. The labeled protein was incubated with immune complexes purified from untreated or tunicamycin-treated (Tm) HT22 cells by means of preimmune sera (PI) or antisera directed to CReP or GADD34. Incubation times were 0, 10, and 20 min (lanes 1, 2, and 3, respectively). The bottom panels are immunoblots of the endogenous CReP and GADD34 from the immunoprecipitates used in the dephosphorylation assay. (D) Autoradiogram of an in vitro dephosphorylation assay of 32P-radiolabeled eIF2α on serine 51. The labeled protein solution was incubated with buffer alone (No lysate) or crude lysate from parental CHO cells or CHO cells stably transduced with the CReP-expressing CD retrovirus described in Fig. 2 (CReP). Reaction times were 10 and 20 min. The asterisk indicates GST-PERK, which is autophosphorylated.