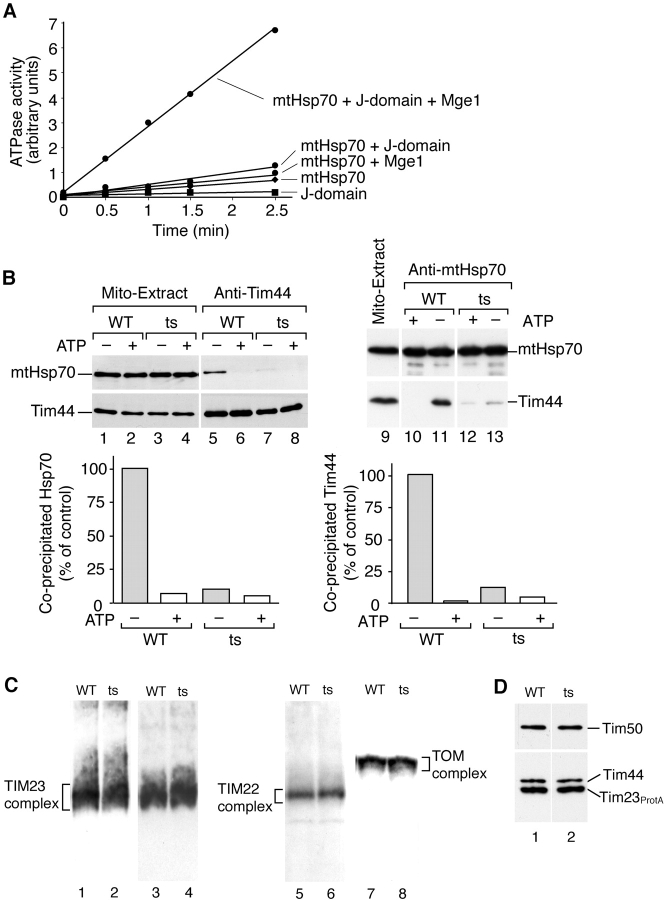
Figure 4.
The J-domain stimulates the ATPase activity of mtHsp70 and influences the Tim44–mtHsp70 interaction. (A) ATPase activity of mtHsp70 under steady-state conditions using [α32P]ATP, purified mtHsp70, Mge1, and the J-domain of the 18-kD protein of PAM. Purified Tim44 did not stimulate the ATPase activity. (B) Disturbance of the Tim44–mtHsp70 interaction in J mutant (ts) mitochondria. Mitochondria were lysed under nondenaturing conditions and subjected to coprecipitation with antibodies directed against Tim44 or mtHsp70 (Voisine et al., 1999). Precipitates were analyzed by SDS-PAGE and immunodecoration (5% of the mitochondrial extracts are shown). Quantification: the amount of coprecipitated protein from wild type under ATP-depleted conditions was set to 100% (control). (C) Blue native PAGE of mitochondria lysed with digitonin, followed by immunodecoration with antibodies directed against Tim23, Tim22, and Tom40. (D) Protein A tagged Tim23 was expressed in wild-type and J mutant cells. Mitochondria were isolated, lysed with digitonin, and subjected to IgG chromatography.