Figure 4.
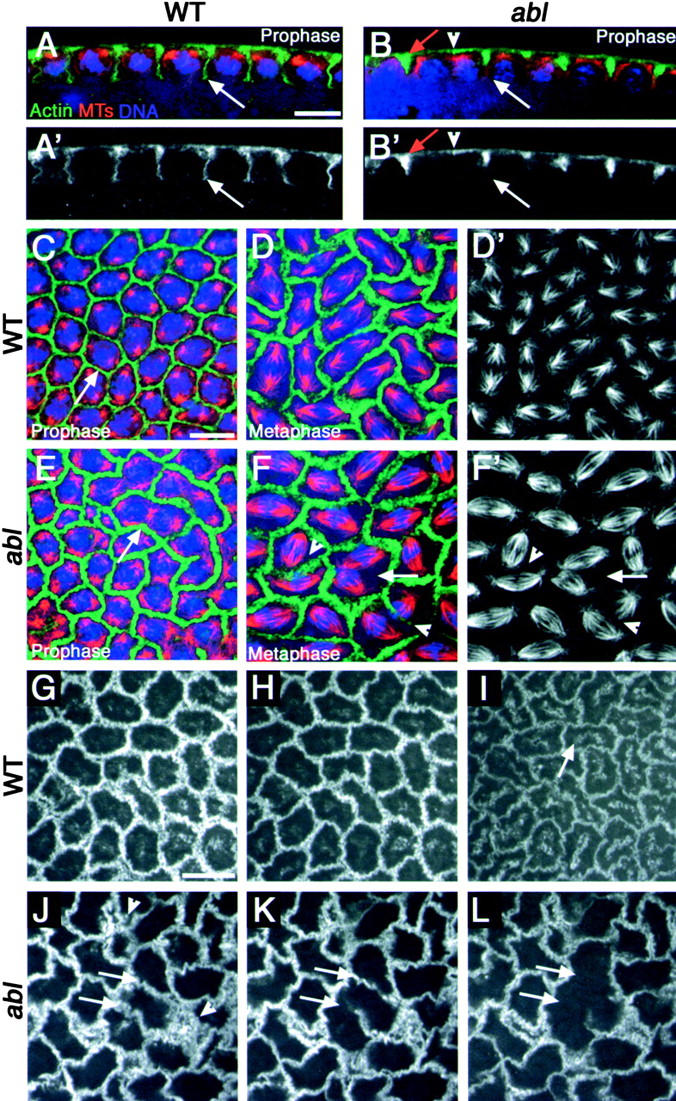
Elevated apical actin localization in ablM mutants is accompanied by furrow defects. (A–F′) Syncytial divisions. F-actin (phalloidin) in green, microtubules in red, and DNA (DAPI) in blue. (A′ and B′) Actin alone. (D′ and F′) Microtubules alone. (A–B′) Cross section. (C–F′) Apical surface view. (A, A′, B, B′, C, and E) Prophase. (D, D′, F, and F′) Metaphase. (A and A′) Wild-type. Pseudocleavage furrows (arrow). (B and B′) ablM. Excess actin in the apical-most region of the furrow (red arrow). Missing furrow (arrowhead). Incomplete furrow (white arrow). (C, D, and D′) Wild-type. (C) Prophase. Pseudocleavage furrows (arrow) are thin and uniform. (D and D′) Metaphase. Furrows thicken as they change shape, and spindles remain separated. (E) ablM. Prophase. The apical-most region of the furrows (arrow) are thicker and more filamentous (compare with C). (F and F′) ablM. Metaphase. Furrow defects lead to spindle collisions both where furrows are absent (arrow) and occasionally where some actin remains (arrowheads). (G–L) Surface view: living embryos expressing moesin-GFP, filmed during syncytial divisions. (G–I) Wild-type (Video 1, available at http://www.jcb.org/cgi/content/full/jcb.200307026/DC1). Prophase (G), metaphase (H), anaphase/telophase (I). Pseudocleavage furrows remain intact. Arrow, Cytoplasmic actin that may be relocalizing to caps. (J–L) ablM (Video 2). Prophase (J), metaphase (K), anaphase/telophase (L). Some furrows break down at anaphase onset (arrows). Excess apical actin (arrowheads). Experiments done at 18°C. Bars, 10 μm.
