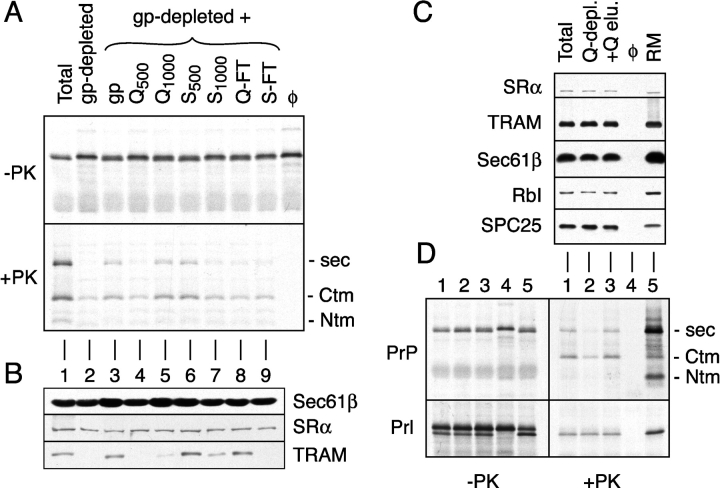
Figure 1.
Detection and fractionation of a translocation accessory factor activity. (A) Analysis of PrP translocation activity in fractionated proteoliposomes. A glycoprotein-depleted detergent extract was mixed with either buffer (lane 2), total glycoproteins (lane 3), or ion exchange fractions of total glycoproteins (lanes 4–9). Proteoliposomes were prepared from each mixture and assayed for their ability to translocate PrP. Shown are translation products before and after digestion with PK. The positions of protease-protected fragments of PrP corresponding to the secPrP, CtmPrP, and NtmPrP forms (Hegde et al., 1998a) are indicated to the right of the autoradiograph. Q-FT and S-FT indicate flowthrough fractions after binding at 200 mM KAc to Q- and S-sepharose, respectively. Fractions resulting from sequential elution of these resins with either 500 mM or 1,000 mM KAc are indicated with a subscript. For comparison, shown are translocation reactions lacking membranes (last lane) and containing proteoliposomes reconstituted from a total unfractionated detergent extract (lane 1). (B) Immunoblots of each of the proteoliposomes from panel A with antibodies against Sec61β, SRα, and TRAM. (C) Proteoliposomes were prepared from a total detergent extract, a detergent extract after depletion of proteins that bind to Q-sepharose (Q-depl.), and a Q-depleted extract replenished with the protein eluted from Q-sepharose (+Q-elu.). Aliquots of each proteoliposome preparation, along with the starting RMs, were immunoblotted with antibodies against the indicated proteins. (D) Translocation of PrP and Prl into the proteoliposomes from panel C. Aliquots of the translation products before and after digestion with PK are shown on the left and right, respectively. Lane 4 is a translocation reaction lacking membranes. The topologic forms of PrP are indicated to the right of the autoradiograph.