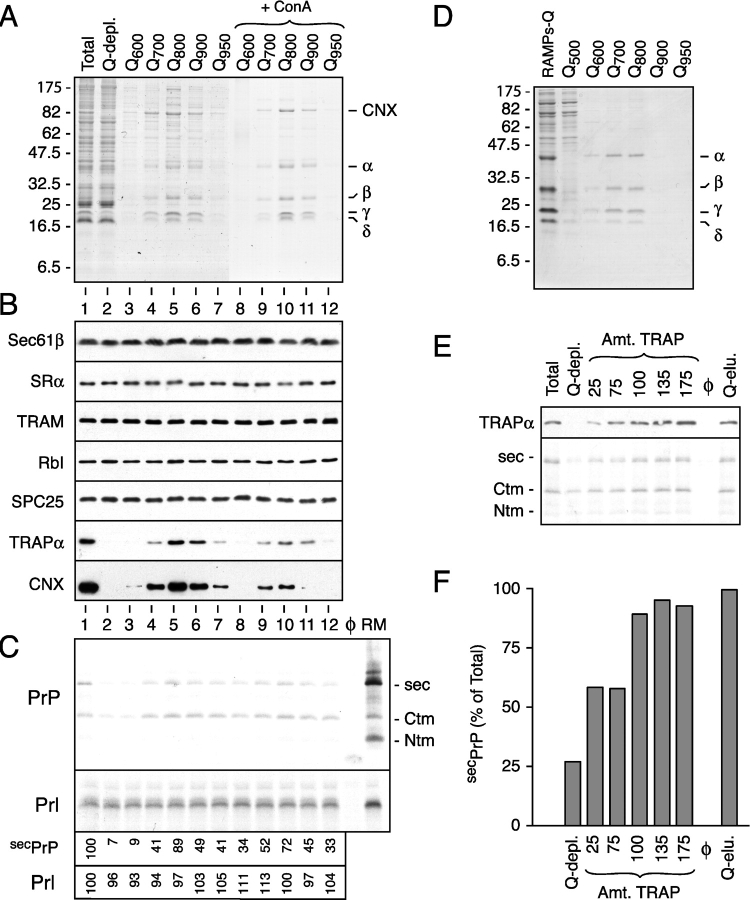
Figure 2.
Purification of TrAF and identification as the TRAP complex. (A) Shown is a Coomassie blue–stained gel of fractions resulting from the separation of membrane proteins by ion exchange and ConA chromatography. The positions of CNX and the α through δ subunits of the TRAP complex are indicated. (B) Immunoblots against various proteins in proteoliposomes prepared using the fractions in panel A. Lanes 1 and 2 contain proteoliposomes reconstituted from the total and Q-depleted detergent extracts, respectively. Lanes 3–12 contain proteoliposomes coreconstituted with the Q-depleted detergent extract plus the respective individual fractions in lanes 3–12 of A. (C) Translocation assays of PrP and Prl using proteoliposomes from panel B. Control reactions lacking membranes or containing RMs are also shown for comparison. Only the translocated material, remaining after digestion of the translation reactions with PK, is shown. The efficiencies of secPrP and Prl translocation, relative to the unfractionated proteoliposomes in lane 1, are shown below the autoradiograph. (D) Purification of the TRAP complex from the RAMP fraction. Shown is the Coomassie blue–stained gel containing the final fractions of the purification. (E) A Q-depleted detergent extract was replenished with varying concentrations of RAMP-purified TRAP or total Q eluate and reconstituted into proteoliposomes. As a control, an unfractionated detergent extract was also reconstituted in parallel. Shown in the top panel is an immunoblot against TRAPα of the different proteoliposomes. The amount of TRAP, as a percent of that found in the unfractionated proteoliposomes, is indicated above the blot. The bottom panel shows the assay for PrP translocation into these proteoliposomes. Only the translocated products remaining after protease digestion are shown. (F) The extent of secPrP translocation in the assay from panel E was quantitated and plotted as a bar graph. The amount of translocation in the Q-depleted proteoliposomes replenished with the total Q-eluate fraction was defined as 100%.