Figure 3.
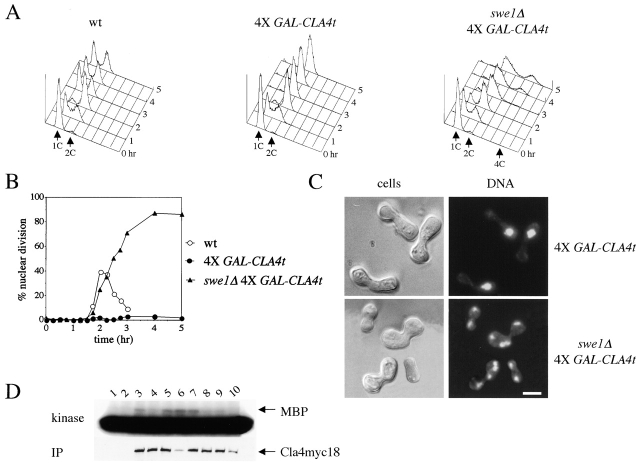
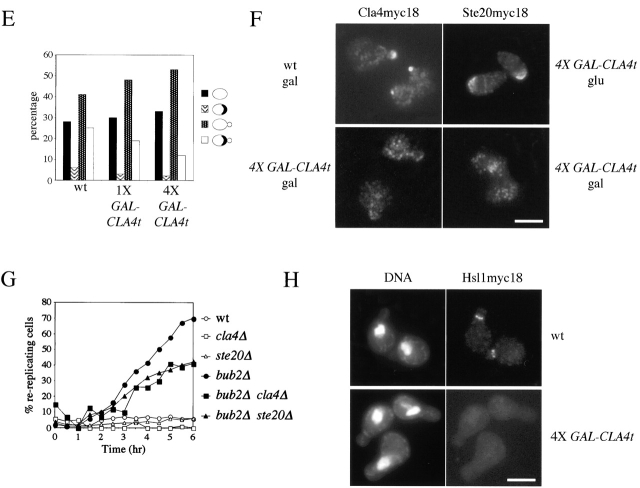
CLA4t overexpression inhibits endogenous Cla4 and Ste20 PAK kinases and causes a Swe1-dependent G2/M arrest. (A–C) Wild-type (W303), 4X GAL-CLA4t (ySP2625) and swe1Δ 4X GAL-CLA4t (ySP2711) cells were grown in YEPR, arrested in G1 with α-factor and then released in YEPRG medium (t = 0). Samples were collected at the indicated times for FACS® analysis of DNA contents (A) and to follow kinetics of nuclear division (B). Photographs were taken at t = 5 h after release (C). Bar, 5 μm. (D) wild-type (ySP3086, lanes 3–6) and 4X GAL-CLA4t cells (ySP3088, lanes 7–10), expressing myc-tagged Cla4 (Cla4myc18), as well as an untagged strain (W303, lanes 1 and 2) were grown in YEPR (lanes 1, 3, and 7) and then shifted to YEPRG for 2 h (lanes 4 and 8) and 3 h (lanes 5 and 9), or to YEPRG with nocodazole for 2.5 h (lanes 2, 6, and 10). Anti-myc immunoprecipitates from the corresponding cell extracts were subjected to Western blot analysis with anti-myc antibodies (IP) and to kinase assays using myelin basic protein (MBP) as substrate. (E) Wild-type (W303), 1X GAL-CLA4t (ySP2622) and 4X GAL-CLA4t (ySP2625) cells carrying a pRS316 plasmid with a GFP-CDC3 fusion (Vallen et al., 2000) were grown in YEPR and then shifted to YEPRG medium. After 3 h, GFP-Cdc3 was scored in unbudded and budded cells (n = 500) to monitor the presence of a septin ring. (F) Left; wild-type (ySP3086) and 4X GAL-CLA4t (ySP3088) cells, expressing Cla4myc18, were grown in YEPR, arrested in G1 with α-factor, and released in YEPRG (t = 0). Samples were collected every 10 min for 2 h for FACS® analysis of DNA contents (not depicted) and immunostaining of Cla4myc18. Photographs were taken at t = 50', when wild-type cells reached a peak of small budded cells with cortical Cla4myc18 (30% of total cells). The fraction of 4X GAL-CLA4t cells with cortical Cla4myc18 staining remained below 1% throughout the time course. Right; 4X GAL-CLA4t cells expressing myc-tagged Ste20 (Ste20myc18, ySP3091) were grown in YEPR, arrested in G1 with α-factor, and then shifted to either YEPD (glu) or YEPRG (gal) medium containing α-factor for 2 h to maintain the G1 arrest. The fraction of cells displaying polarized localization of Ste20 was 55% in YEPD and 3% in YEPRG (n = 100). Bar, 5 μm. (G) Wild-type (W303), cla4Δ (ySP3076), ste20Δ (ySP3078), bub2Δ (ySP3138), bub2Δ cla4Δ (ySP3186), and bub2Δ ste20Δ (ySP3198) cells were grown in YEPD, shifted to YEPD containing nocodazole (t = 0), and collected at the indicated times for FACS® analysis of DNA contents. The percentage of re-replicating cells at each time point was calculated as described in Materials and methods. (H) Wild-type (ySP3157) and 4X GAL-CLA4t (ySP3202) cells expressing myc-tagged Hsl1 were grown in YEPR, arrested in G1 with α-factor, and released in YEPRG. Samples were collected at different times for immunostaining of Hsl1myc18. Photographs were taken 90' after release. Bar, 5 μm.