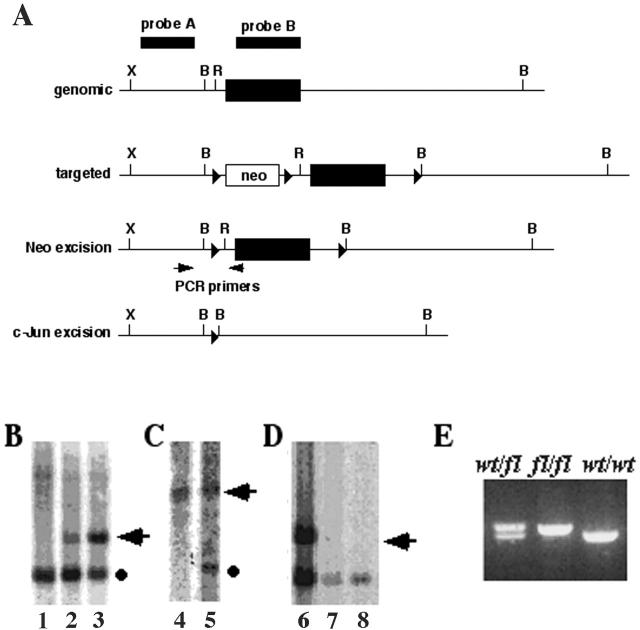
Figure 1.
The generation of floxed c-jun mice. (A) Schematic diagrams of the c-jun gene, the targeted locus, and the final floxed allele with the neo cassette excised and the null allele resulting from Cre-mediated excision of c-jun. The probes used for screening the Southern blots for detecting the insertion of the neo cassette (probe A) and to assess the incorporation of the 3′ loxP and BamHI site (probe B) are indicated, as are the PCR primers used for detecting the incorporation of the 5′ loxP site. (B) wt (lane 1) or 2 lines of targeted ES cell (lanes 2 and 3) DNA was digested with XbaI-EcoRI and hybridized with probe A, which detects a fragment of 1.9 kb (circle) from wt, and 2.8 kb (arrowhead) from the targeted allele containing the neo gene. (C) The 3′ loxP site is intact, as shown by the presence of the adjacent BamHI site. DNA from wt ES cells (lane 4) or a targeted clone (lane 5) was digested with BamHI and hybridized with probe B to reveal a 4.9-kb band (arrowhead) from the wt locus or a 2.2-kb band (circle) from the targeted allele. (D) The neo gene was excised by transfection with pCMV-Cre. DNA from a targeted line (the same as lane 2) before (lane 6) and after (lanes 7 and 8) transfection with Cre was digested with XbaI-EcoRI and hybridized with probe A. The 1.9-kb band is the same in the wt and excised allele, whereas the loss of the 2.7-kb band (arrowhead) indicates removal of neo to give the final floxed c-jun allele. (E) Evidence for germline transmission of the floxed allele was obtained using primers flanking the loxP sites to amplify DNA from c-jun fl/wt, c-jun fl/fl, and wt mice.