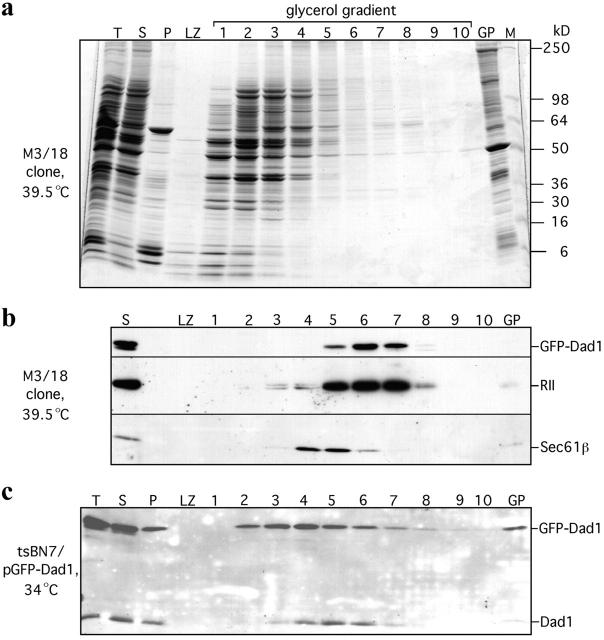
Figure 3.
In detergent extracts from M3/18 cells grown at the nonpermissive temperature GFP–Dad1 cosediments in a glycerol gradient with the other subunits of the OST complex. M3/18 cells grown at 39.5°C were solubilized with 1.5% of digitonin in the presence of 0.5 M NaCl. The total cell lysate (T) was clarified by differential centrifugation obtaining a supernatant (S) and a pellet fraction (P). An aliquot (0.85 ml) of the supernatant fraction was layered onto a glycerol gradient (8%-30%) and after centrifugation (151,200 g for 15.5 h) the gradient was fractionated into ten 1.15-ml fractions and the LZ. Equal aliquots of the gradient fractions, the pellet formed during centrifugation (GP) as well as molecular weight markers (M) were analyzed by SDS-PAGE. The proteins on the gel were either stained with Coomassie blue (a) or transferred onto a nitrocellulose membrane and probed by Western blotting with pAbs against Dad1, RII and Sec61β (b). At the experimental conditions chosen, the OST complex (GFP–Dad1 and RII) and the Sec61 complex (Sec61β) do not cosediment. (c) In a similar experiment, tsBN7 cells transiently overexpressing GFP–Dad1 and grown at 34°C (tsBN7/pGFPDad1) were solubilized with digitonin and analyzed by Western blotting as described above. Using pAb against Dad1 to localize Dad1 and GFP–Dad1 on the glycerol gradient it is apparent, that most of the overexpressed GFP–Dad1 was not incorporated into the OST.