Figure 1.
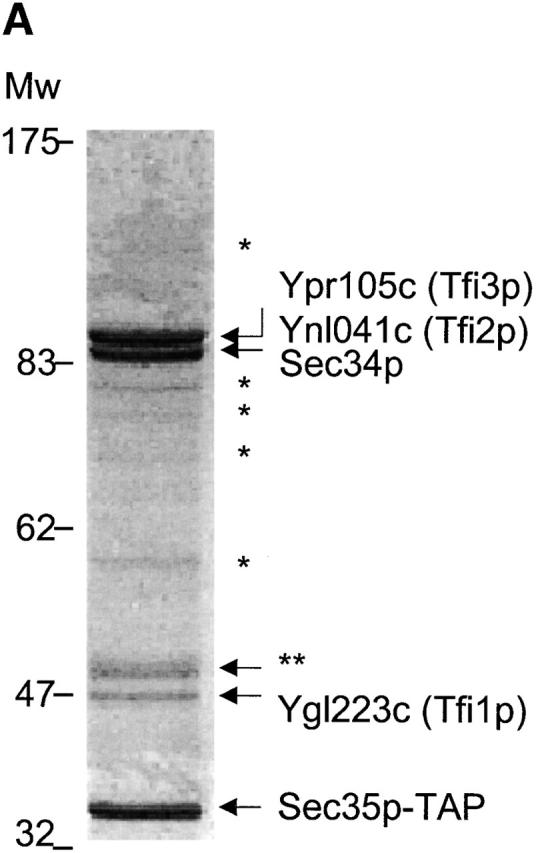
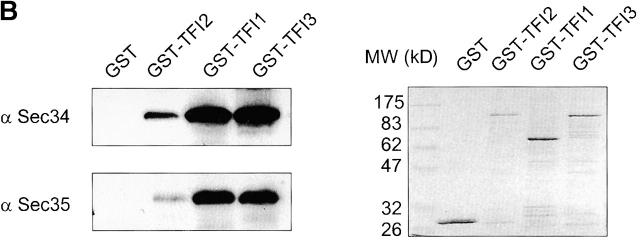
Sec35p-TAP complex purified from yeast cytosol. (A) Sec35p-TAP is associated with four other proteins. Proteins eluted from calmodulin-agarose beads were separated by gel electrophoresis and stained with Coomassie blue. The indicated bands were identified by mass spectroscopy of tryptic fragments. The band labeled with double asterisks represents a proteolytic fragment of Tfi3p. No readable spectra were obtained from the minor bands labeled with asterisks. (B) GST-tagged TFI1, TFI2, and TFI3 interact with both Sec34p and Sec35p. The GST-tagged proteins were expressed in cells of appropriate gene deletion strains, in which the GST-chimera was the only source of TFI1, TFI2, or TFI3 protein. Membrane fractions were obtained after centrifugation at 150,000 g, 1 h, 4°C. Extracted membrane proteins (2 mg) were incubated with 50 μl prewashed glutathione–Sepharose beads. The eluates were loaded on the 10% SDS-PAGE and then analyzed by immunoblot with α Sec34p and α Sec35p (left), or stained with Coomassie blue (right).