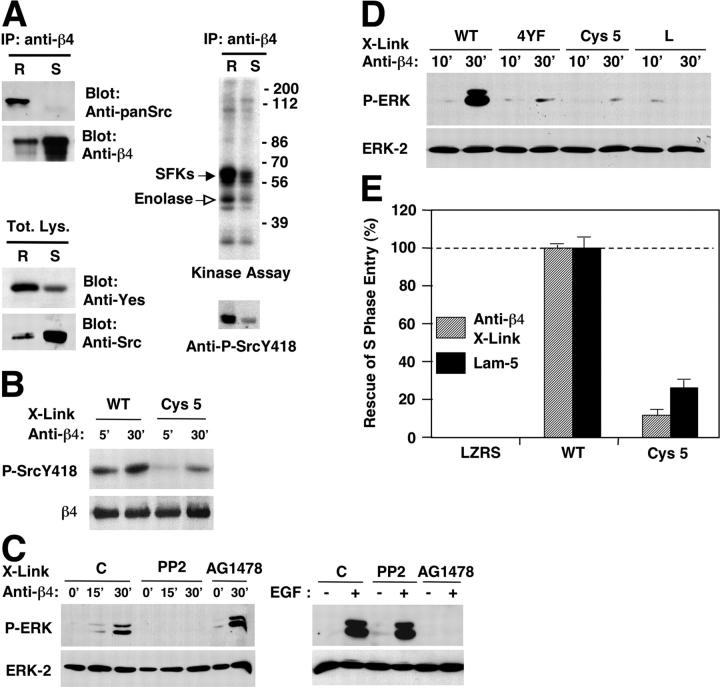
Figure 4.
Compartmentalization of α6β4 signaling in lipid rafts. (A) After antibody-mediated ligation of α6β4, HaCat cells were lysed with Triton X-100 and subjected to OptiPrep™ gradient ultracentrifugation. The lipid raft (R) and Triton X-100–soluble (S) fractions were either immunoprecipitated with mAb 3E1 and probed with anti-β4 or anti-pan-Src, or directly probed with antibodies reacting with Yes or Src. The lipid raft and Triton X-100–soluble fractions were also immunoprecipitated with mAb 3E1 and subjected to kinase assay or immunoblotting with anti-phospho Src Y418. (B) PA-JEB keratinocytes expressing wild-type β4 or β4 Cys 5 were plated on dishes coated with mAb 3E1 for the indicated times. Total lysates were probed with anti-phospho Src Y418 or anti-β4 N20. (C) HUVECs transiently transfected with vectors encoding α6β4 were left untreated (C) or treated with the SFK inhibitor PP2 or the EGF-R inhibitor AG1478. Cells were plated on dishes coated with mAb 3E1 for the indicated times or stimulated with EGF. Total proteins were probed with anti-phospho-ERK and anti-ERK-2. (D) HUVECs were transiently transfected with α6 in combination with wild-type β4, phosphorylation-defective β4 (4YF), β4 Cys 5, or tail-less β4 (L). Cells were plated for the indicated times on dishes coated with mAb 3E1. Blotting was as in B. (E) PA-JEB keratinocytes stably transduced with empty vector (LZRS), wild-type β4, and β4 Cys 5 were deprived of mitogens, detached, and plated on coverslips coated with purified laminin-5 or incubated in suspension with mAb 3E1, and were then plated on coverslips coated with anti–mouse IgGs. After 24 h of incubation with EGF, the cells were subjected to anti-BrdU staining. The data represent the average and SDs of values obtained from three experiments and are expressed as percentage of rescue.