Figure 7.
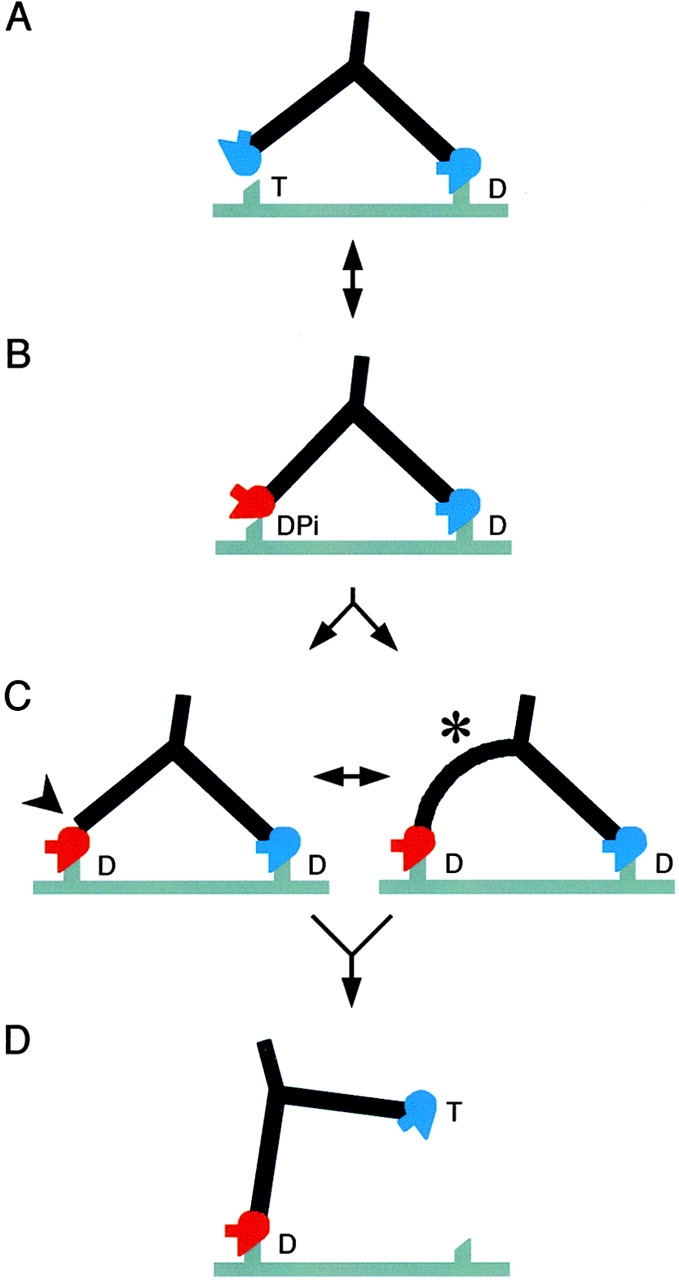
Structural cycle of myosin V walking. Red motor domains represent the converter and lever in the prepower stroke conformation, and blue ones represent the postpower stroke conformation. The actin filament is gray and shows two preferred attachment sites for heads spaced by 36 nm. Myosin (drawn to a similar scale) is walking left and has a head orientation that shows a prominent SH3 domain. T, DPi, and D (ATP, ADP + phosphate, and ADP) indicate the likely head nucleotide contents in vivo. Note that if ATP is limiting in vitro, as in our data with actin, then the head contents will be different (see Discussion). (A) The attached head is close to the rigor orientation; the motor domain of the second head can reach the next binding site on actin, but the apo-like head shape is wrong for attachment. (B) The detached head has switched to the angled conformation seen in unattached molecules (Fig. 4 B), which in this view appears straight (Fig. 5 B, scallop–ADP.VO4). The motor domain may attach weakly to actin, but is not oriented correctly for strong binding because the lever is not perpendicular to the actin filament. (C) Stereospecific attachment of the lead head motor to actin. We propose that attachment is achieved by distortion either at the lead head pliant region (arrowhead), or throughout its lever (asterisk). This explains why both straight and curved levers can be seen in lead head images, even though the converters are in the prepower stroke position. Note that little forward movement of the myosin tail accompanies attachment of the lead head. For simplicity, the subsequent actin-activated release of Pi is not shown as a separate step. Note also that though both heads contain only ADP, they have very different conformations. (D) Release of the trail head on binding ATP. The intramolecular strain is relieved, the lead lever becomes nearly perpendicular to the filament and the myosin tail moves forward. This would quickly be followed by the working stroke of the lead head, completing the cycle.
