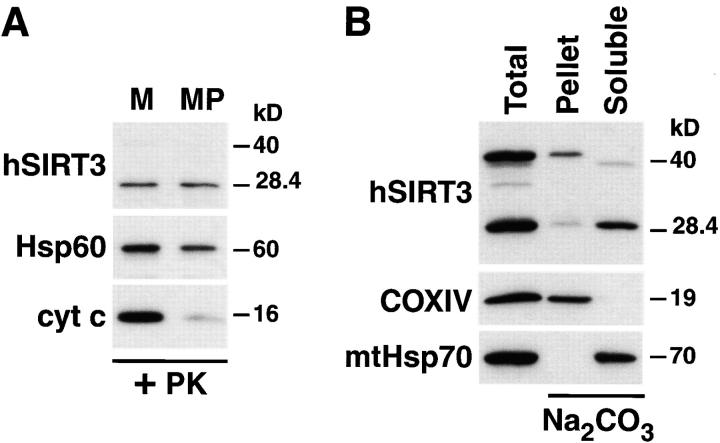
Figure 5.
hSIRT3 is localized in the mitochondrial matrix. (A) Mitochondria were isolated from HEK293T cells transfected with hSIRT3–FLAG and treated with proteinase K to remove proteins bound to the outer mitochondrial surface. Mitochondrial preparations were divided, and one half was diluted with hypotonic buffer to create mitoplasts (MP), while the other half was maintained under isotonic conditions (M). After incubation (20 min at 0°C), mitochondria and mitoplasts were treated again with proteinase K and reisolated by centrifugation followed by Western blotting. Rupture of the outer mitochondrial membrane was confirmed by detection of endogenous intermembrane space protein cytochrome c (cyt c). Integrity of the inner mitochondrial membrane was determined with the matrix protein Hsp60 as a marker. hSIRT3-FLAG was detected using anti-FLAG M2 antibodies. (B) Mitochondria were isolated from HEK293T cells transfected with hSIRT3–FLAG and treated with proteinase K. The preparation was divided, and one half was resuspended in SDS sample buffer (Total, left lane). The other half of the preparation was resuspended in sodium carbonate (Na2CO3) buffer. The extract was centrifuged at 100,000 g at 4°C, and the mitochondrial membranes (Pellet, middle lane) were resuspended in SDS sample buffer. The supernatant containing the soluble and peripheral membrane proteins (Soluble, right lane ) was precipitated with TCA. Samples were analyzed by Western blotting. hSIRT3 was detected with anti–FLAG antibodies. Alkaline extraction was controlled by detection of the marker proteins COXIV and mtHsp70.