Figure 1.
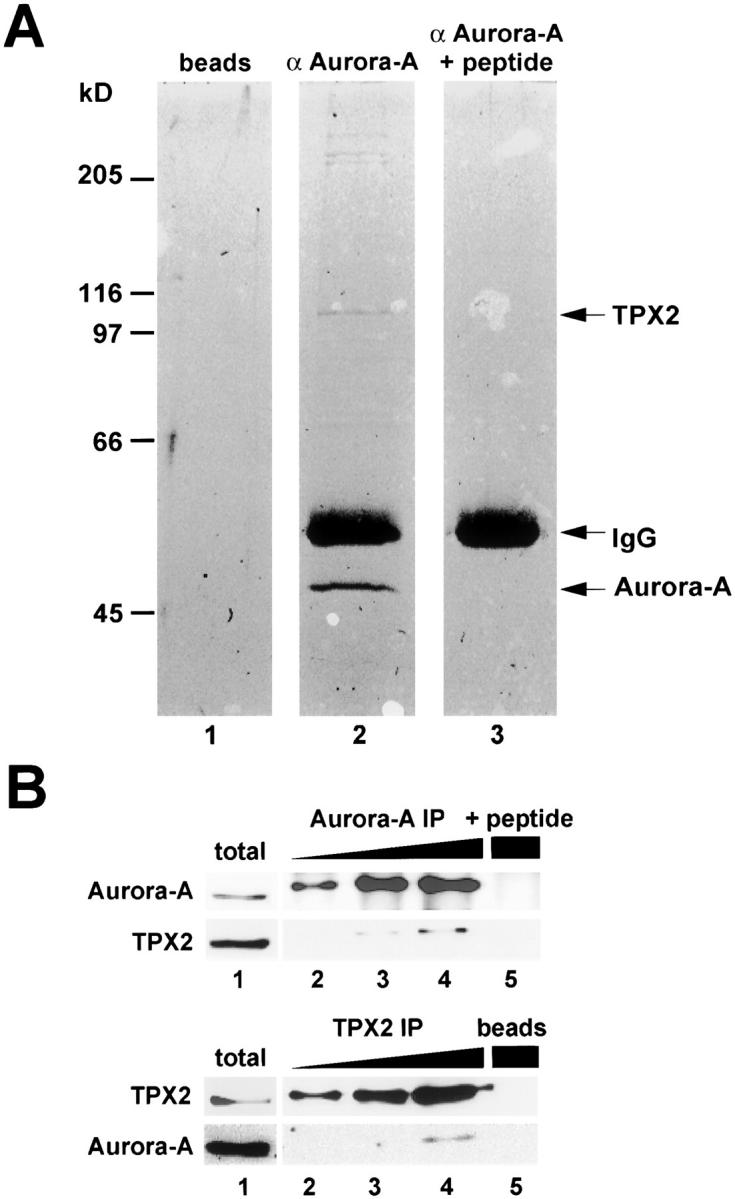
Identification of TPX2 as an Aurora-A–interacting protein. (A) Coomassie blue–stained gel of Aurora-A immunoprecipitate from mitotic HeLa cell extract (lane 2). For control, immunoprecipitations were also performed with either protein A–coated beads alone (lane 1) or Aurora-A antibody blocked with an excess of antigenic peptide (lane 3). Arrows point to the precipitated 45-kD Aurora-A protein, IgG heavy chain, and the 100-kD protein identified as TPX2 by tryptic peptide fingerprinting. The identity of the high molecular weight proteins coprecipitating with Aurora-A is currently under investigation. (B, top) Increasing amounts of Aurora-A immunoprecipitates (lanes 2–4) were separated by SDS-PAGE and analyzed by Western blotting using antibodies against Aurora-A and TPX2. The control immunoprecipitation was performed with the peptide blocked Aurora-A antibody (lane 5). (B, bottom) TPX2 coimmunoprecipitates were analyzed by Western blotting using antibodies against Aurora-A and TPX2. Beads alone were used for the control precipitation (lane 5). Total cell lysate was analyzed in parallel (lane 1).
