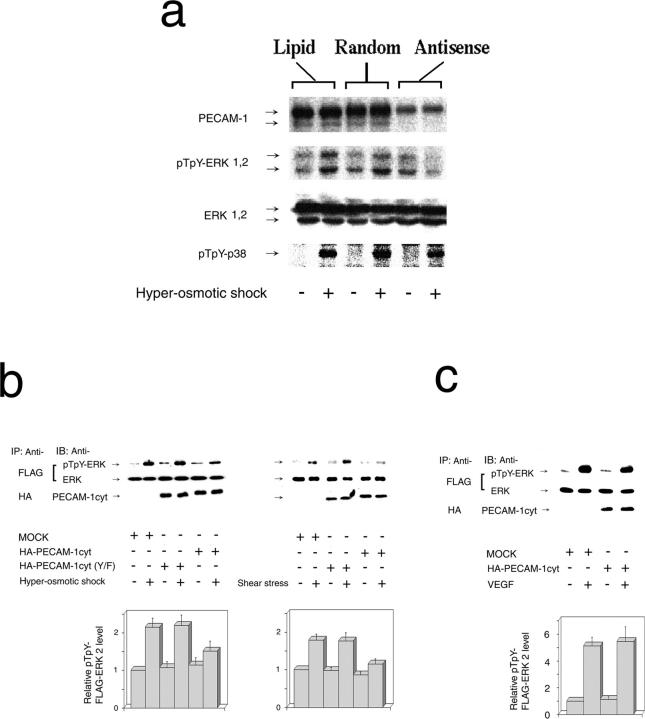
Figure 2.
ERK activation by mechanical stresses depends on the presence and tyrosine phosphorylation of PECAM-1. (a) BAECs were treated with PECAM-1 antisense S-oligo (Antisense), control scrambled S-oligo (Random), or lipid carrier (Lipid) using the high transfection efficiency condition. Cells were grown to confluency and treated with (+) or without (−) HOS for 10 min. Cell lysates were immunoblotted with anti–PECAM-1 (PECAM-1), anti–phospho-ERK (pTpY-ERK1,2), anti-ERK (ERK1,2), and anti–phospho-p38 (pTpY-p38). PECAM-1 downregulation inhibited ERK, but not p38, phosphorylation by HOS. The data are from one of five sets of similar experiments. (b) FLAG–ERK2 was transiently coexpressed with HA– PECAM-1cyt or HA–PEAM-1cyt (Y/F) in BAECs. Confluent transfectants were treated with (+) or without (−) HOS (left) or FSS (right) for 10 min. Cell lysates were immunoprecipitated (IP) with anti-FLAG (FLAG) or anti-HA (HA) followed by immunoblotting (IB) with anti–phospho-ERK (pTpY-ERK), anti-ERK (ERK), or anti–PECAM-1 (PECAM-1cyt). Note that PECAM-1cyt, but not PECAM-1cyt(Y/F), has a dominant negative effect. (c) BAECs coexpressing FLAG–ERK and PECAM-1cyt were treated with 50 ng/ml of VEGF for 10 min. PECAM-1cyt did not inhibit ERK activation by VEGF. Control cells were transfected with FLAG–ERK and lipid carrier (MOCK) and treated similarly with VEGF. Bar graphs in b and c show relative FLAG–ERK2 phosphorylation levels (mean ± SEM) of 4–12 experiments.