Figure 4.
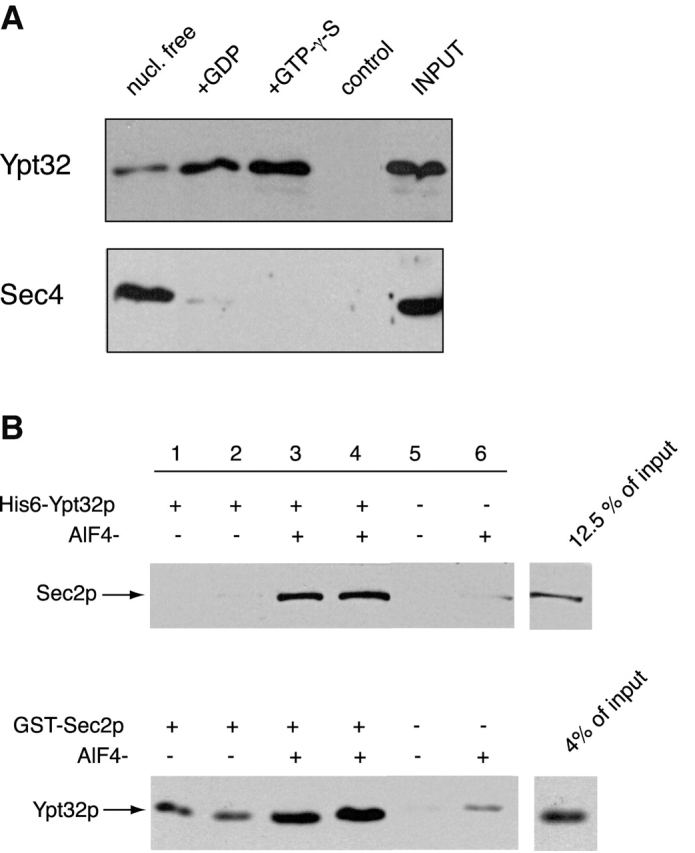
Sec2p binds to Ypt32p with higher apparent affinity for its activated form. (A) GST–Sec2p immobilized on glutathione-Sepharose beads was incubated with Ypt32p as described in the Materials and methods. Ypt32p was either stripped of nucleotide (nucleotide free, lane 1) or loaded with GDP (lane 2) or GTP-γ-S (lane 3). Control (lane 4) represents Ypt32p binding to the GST protein immobilized on glutathione-Sepharose beads. Input represents 5% (1.5 ng) of total Ypt32p used in binding mixtures. An identical experiment was performed for Sec4p. Input represents 20% (6 ng) of Sec4p protein used per binding tube. The beads were washed and the bound proteins were eluted with SDS-PAGE sample buffer. The eluates were then resolved on an SDS-PAGE gel, and Western blot analysis was performed using αYpt32p and αSec4p antibodies. (B) In the top panel, His6–Ypt32p (1 μM) was incubated with Ni-NTA beads and a portion of lysate, prepared from yeast overexpressing GST–Sec2p in a buffer containing PBS and 10 mM imidazole with or without 50 μM AlCl3 and 10 mM NaF. After incubation for 60 min at 4°C, the beads were washed and the bound proteins were analyzed by SDS-PAGE. GST–Sec2p was detected by αSec2p antibody. In the bottom panel, GST–Sec2p (10 nM) immobilized on glutathione-Sepharose beads was incubated with His6–Ypt32p (3 nM) in a buffer containing PBS and 1 mg/ml BSA with or without 50 μM AlCl3 and 10 mM NaF. Bound Ypt32p was detected by αYpt32p antibody.
