Figure 2.
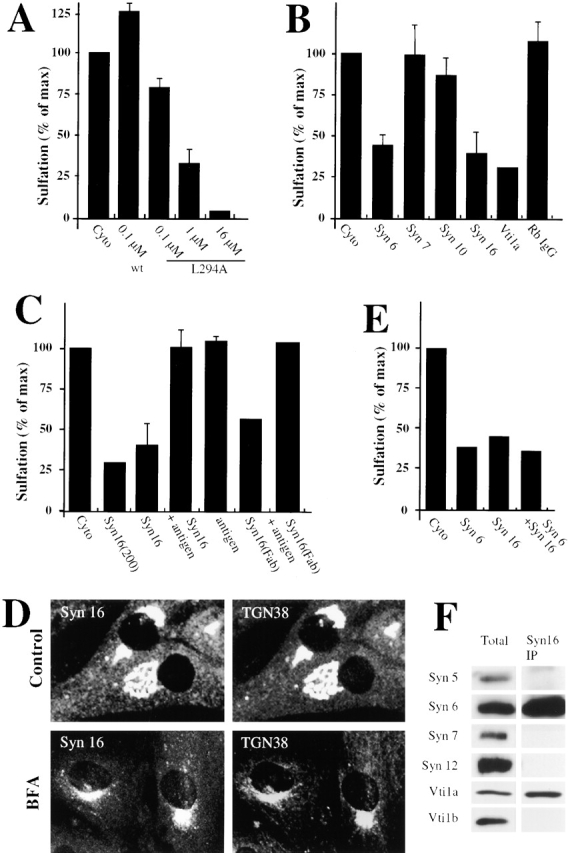
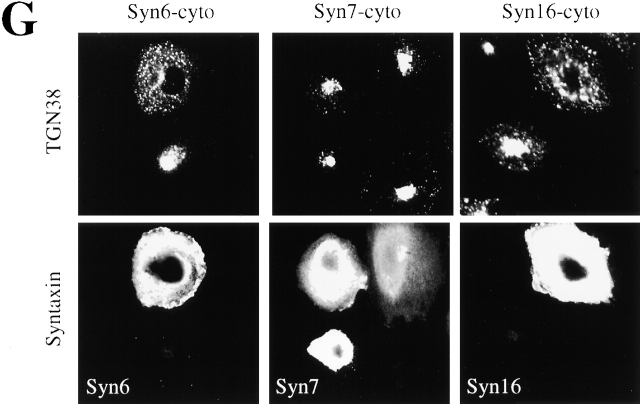
Retrograde transport to the TGN is mediated by the t-SNAREs Syn6, Syn16, and Vti1a. An experimental protocol as shown in Fig. 1 A was used. (A) STxB-Sulf2 transport to the TGN was assayed by sulfation analysis in the presence of the indicated concentrations of recombinant wild- type α-SNAP (wt) or a dominant negative α-SNAP mutant (L294A). As in the following parts of the figure, means (± SEM) of two to six experiments are shown. (B) 25–50 μg/ml of anti-Syn6, 7, 10, 16, or anti-Vti1a antibodies were continuously present from permeabilization on. Rb IgG, rabbit control IgG. The experiments with Syn6 were performed both with a monoclonal and a polyclonal antibody. (C) Anti-Syn16 antibody and Fab fragments generated from this antibody (Syn16[Fab]) had comparable inhibitory effects on STxB-Sulf2 transport to the TGN. Inhibition could be reversed by prebinding of the antibodies to recombinant His-tagged Syn16. Higher doses of anti-Syn16 (200 μg/ml; Syn16[200]) did not significantly increase the inhibitory effect. (D) Syn16 localization in the TGN. Note that upon BFA treatment, the perinuclear staining of TGN38 and Syn16 collapsed into a microtubule organizing center-like staining, a characteristic of TGN proteins. (E) Antibodies against Syn6 and Syn16 had no additive inhibitory effects on STxB-Sulf2 transport to the TGN, suggesting that both proteins function in the same molecular complex. (F) Antibody against Syn16 coimmunoprecipitated Syn6 and Vti1a, but not Vti1b, the cis-Golgi Syn5 or the endosomal Syn7 or Syn12. (G) Expression of Syn6-cyto and Syn16-cyto, but not of Syn7-cyto in Tac-TGN38–transfected CHO cells, specifically inhibited transport of internalized anti-Tac antibodies to the TGN.