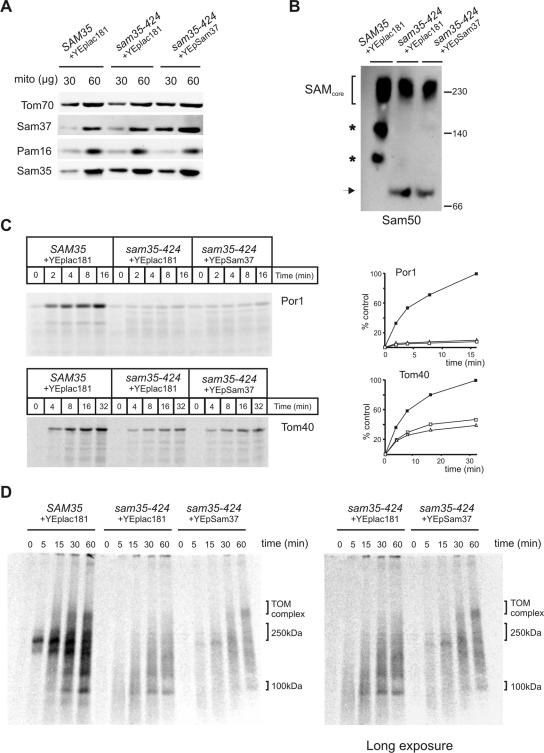
Figure 3.
Overexpression of Sam37 suppresses the phenotypes of sam35-424 by functioning as an assembly factor downstream of Sam35. (A) Mitochondria from Δsam35 cells transformed with plasmid containing wild-type SAM35 and vector YEplac181, sam35-424 transformed with the vector YEplac181, and sam35-424 expressing multiple copies of SAM37 from YEplac181 were analyzed by SDS-PAGE, and immunoblot analysis was completed using antisera against the indicated proteins. (B) Mitochondria (100 μg) from wild-type SAM35 or sam35-424 cells, transformed with the indicated plasmids, were solubilized in 1% digitonin buffer. Protein complexes analyzed by BN-PAGE, followed by blotting onto PVDF membrane and immunodecoration by using anti-Sam50 antisera. Asterisks, unidentified subcomplexes of the SAM complex obtained during solubilization; arrow, Sam50-containing complex specifically enriched in sam35-424. (C) Mitochondria from the indicated strains were preincubated at 37°C for 15 min, followed by equilibration at 25°C for 5 min before incubation with 35S-labeled porin or Tom40 at 25°C for the indicated time. Unimported precursors were removed by treatment with 50 μg/ml proteinase K, and imported proteins were analyzed by SDS-PAGE and digital autoradiography. Quantification of the results shown on the right. Filled boxes, SAM35; open boxes, sam35-424; open triangles, sam35-424 overexpressing SAM37. (D) 35S-labeled Tom40 was imported. into mitochondria from the indicated strains. Shown on the right is a longer exposure of a portion from the same gel.