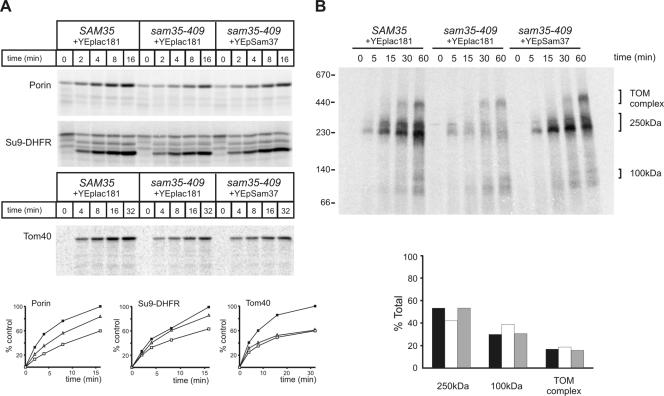
Figure 5.
Restoration of the TOM and SAM complexes in sam35-409 by overexpression of Sam37 cure import and assembly defects into various mitochondrial subcompartments. (A) 35S-labeled porin, Su9-DHFR, and Tom40 were incubated with mitochondria isolated from Δsam35 cells transformed with plasmid encoding wild-type SAM35 and vector YEplac181, sam35-409 transformed with the vector YEplac181, and sam35-409 expressing multiple copies of SAM37 from YEplac181 for the indicated time at 25°C. Precursors not imported were removed by treatment with 50 μg/ml proteinase K and analyzed by SDS-PAGE, followed by digital autoradiography. Quantification of the results is shown below. Filled boxes, SAM35; open boxes, sam35-409; open triangles, sam35-409 overexpressing Sam37. (B) 35S-labeled Tom40 precursor was incubated with the mitochondria from the indicated strains for the indicated time at 25°C. Mitochondria were reisolated and solubilized in 0.8% digitonin buffer. Insoluble materials were pelleted, and protein complexes were analyzed by BN-PAGE and digital autoradiography. Quantification of the signals from each assembly intermediate as a percentage of the total signal from 250-kDa, 100-kDa intermediates, and the TOM complex is shown below. Black bars, SAM35; white bars, sam35-409; gray bars, sam35-409–overexpressing Sam37.