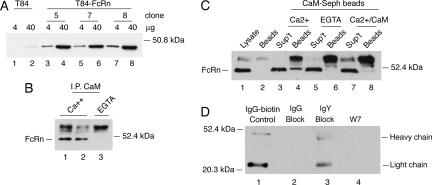
Figure 7.
Calmodulin binds to FcRn expressed in human T84 cells and regulates FcRn-dependent IgG transcytosis. (A) T84 cells (lanes 1 and 2) and T84 cells overexpressing human FcRn (T84-FcRn clones 5, 7, and 8; lanes 3–8) were lysed and examined for FcRn expression by immunoblot. (B) T84-FcRn cell lysates containing calcium (lanes 1 and 2) or EGTA (lane 3) were incubated with protein G-Sepharose beads covalently coupled to an anti-calmodulin mAb. FcRn bound to the beads was detected by immunoblot. Nonspecific bands associated with the protein G-Sepharose beads cross-react with the rabbit antiserum and are shown in each lane above the 52.4-kDa molecular weight marker. (C) T84-FcRn cell lysates containing calcium (lanes 3 and 4), EGTA (lanes 5 and 6), or excess bovine brain calmodulin (lanes 7 and 8) were incubated with calmodulin-Sepharose beads. FcRn bound to the beads (lanes 4, 6, and 8) or retained in the nonbinding fraction (sup't) were detected by immunoblot. FcRn present in whole cell lysates is shown as a control in lane 1. A high-molecular-weight cross-reactive band released from calmodulin-Sepharose beads is shown in lane 2 and in each sample containing beads (lanes 4, 6, and 8). (D) Basolateral-to-apical transcytosis of biotinylated human IgG (IgG-biotin) was examined in T84 cells pretreated with excess underivatized human IgG (lane 2), chicken IgY (lane 3), or W-7 (lane 4) before addition of ligand. Transport of IgG-biotin into the apical buffer was assessed by avidin-HRP immunoblot. Ligand is shown in lane 1 as a control. Results are representative of four independent experiments.