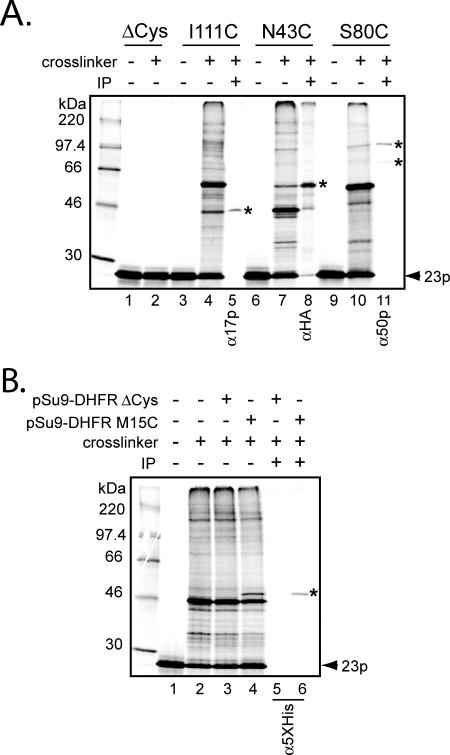
Figure 2.
Cross-linking and immunoprecipitation of Tim23p monocysteine mutants. (A) After [35S]Tim23p monocysteine mutants were imported into isolated mitochondria (either D273-10B, or mitochondria from the strain expressing Tim23p-HA [lanes 6–8]), samples were treated either with the cross-linking reagent BMOE (+cross-linker) or with solvent only (−cross-linker), as indicated. Cross-linked samples were then used for immunoprecipitation (lanes 5, 8, and 11) using antiserum against Tim17p, HA, and Tim50p (α17p, αHA, and α50p, respectively). Cross-linked adducts identified by immunoprecipitation are indicated by asterisks. Other cross-linked bands were not identified. (B) Imported Tim23p S34C forms a cross-linked adduct with the pSu9-DHFR translocation intermediate. After import of [35S]Tim23p S34C into isolated mitochondria, samples were incubated with either 1 μM pSu9-DHFR ΔCys (lanes 3 and 5), 1 μM pSu9-DHFR M15C (lanes 4 and 6), or protein buffer only (lanes 1 and 2). Samples were then treated with BMOE (lanes 2–6) or solvent only as shown. Immunoprecipitation of selected samples (lanes 5 and 6) was done using antibodies against the pentahistidine epitope, which recognizes the affinity tag on the pSu9-DHFR proteins. The cross-link between Tim23p and substrate is identified by the asterisk.