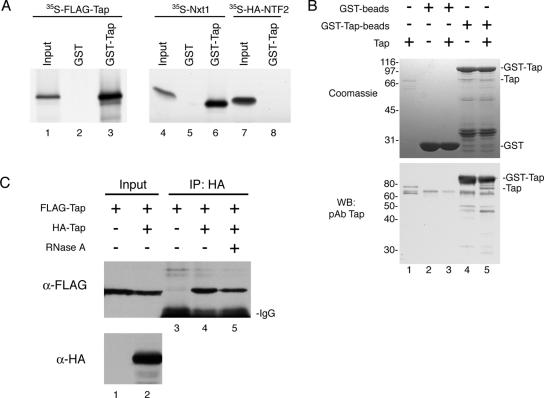
Figure 1.
Complex formation of human Tap in vitro and in vivo. (A) In vitro translated 35S-FLAG-Tap (lanes 1–3), 35S-Nxt1 (lanes 4–6), and 35S-HA-NTF2 (lanes 7 and 8) were incubated with GST alone (lanes 2 and 5) or with GST-Tap (lanes 3, 6, and 8) immobilized on glutathione-coated agarose beads. Bound proteins were detected by autoradiography. Input lanes represent 5% of total 35S-labeled protein added to each reaction. (B) In a similar assay, 2 μg of recombinant Tap (input; lane 1) was assayed for binding to GST (input; lane 2) or GST-Tap beads (input; lane 4). Bound protein (lanes 3 and 5) was loaded onto an SDS-PAGE gel and detected by Coomassie Blue R250 staining (top) or by Western blotting by using the Tap pAb (bottom). (C) Co-IP of FLAG-Tap from COS-7 cells expressing FLAG-Tap alone (lane 1) or FLAG-Tap with HA-Tap (lane 2). Protein complexes were immunoprecipitated using the HA.11 mAb, and the presence of FLAG-Tap bound to HA-Tap was detected by Western blotting (lanes 3–5). Some of the FLAG-Tap + HA-Tap cell extract was pretreated with RNase A before IP (lane 5).